Release date: 2016-01-29
Mitochondria is a subcellular organelle that is widely found in animal and plant cells and is the main site for cellular aerobic respiration and energy production. Hydrogen peroxide (H2O2) is an important reactive oxygen species, mainly produced in the cellular mitochondrial aerobic respiratory electron transport chain. H2O2 is involved in the process of redox and signal transduction in vivo, but the excessive accumulation of H2O2 in the cell causes metabolic disorders in the organism, leading to a series of diseases such as cancer, diabetes, Parkinson's disease and the like. Fluorescent probes and imaging technologies with selective recognition play an important role in real-time-dynamic-visual detection of biologically active substances, and are widely used in many fields such as biology, medical treatment, and clinical diagnosis. However, fluorescent probes that can be accurately localized to the mitochondria of cells to achieve specific detection of H2O2 are still scarce.
Funded by the National Natural Science Foundation of China (No. 21505145, 21572239) and the Gansu Provincial Outstanding Youth Fund Project (No. 1210RJDA013), the research team led by Shao Shijun, a researcher at the Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences, has been working on synthetic receptors for many years. Molecular design and its identification and chemical sensing research. Recently, the research group synthesized a mitochondrial target with a quinoline group functionalized with methylphenylboronic acid as a mitochondrial targeting and H2O2 recognition unit, and a carbazole group as a signal reporting unit. H2O2 fluorescent probes with functional, excellent biocompatibility and OFF-ON fluorescence response.
The probe is capable of detecting H2O2 with high sensitivity and high selectivity, and has a short response time (<5 min) and a low detection limit (0.04 μM). Combined with laser confocal imaging technology, the probe was successfully applied to the detection of H2O2 in human cervical cancer cells (HeLa). The probe molecule enables targeted localization of mitochondria and rapid, highly sensitive, specific detection and imaging analysis of exogenous and endogenous H2O2 in tumor cell mitochondria.
This work has developed a novel molecular design strategy for mitochondria-targeted fluorescent probes, which is expected to be applied in the fields of disease warning, drug development and bioimaging related to reactive oxygen species detection. Related work is published in Analytical Chemistry, 2016, 88(2), 1455-1461.


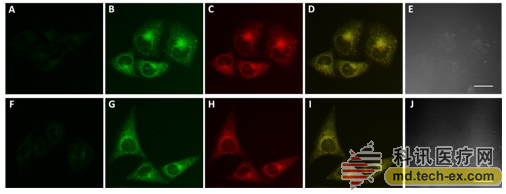
Â
Source: Lanzhou Institute of Chemical Physics
operation in China.
Intranasal spray administration, no injection and no pain
Produced with SPF embryonated eggs
pollution is excluded.
Influenza Vaccine,Influenza Vaccine Development,Influenza Vaccine Strains,Flu Vaccine
Changchun BCHT Biotechnology Co. , https://www.ccbcht.net