Medical Network July 16th With the popularity of "I am not a drug god", the public's attention and discussion on the pharmaceutical industry, innovative drugs, drug prices, medical insurance payment has reached an unprecedented level, whether for the country or enterprises, innovative drugs The development is getting more and more attention. Statistics from the Rubik's Cube database show that as of July 4, 2018, in the first half of 2018, CDE hosted a total of 338 innovative drug acceptance numbers, involving 197 different varieties, articles from product diseases, hot areas and companies to the first half of 2018. The types of declarations worthy of attention were analyzed.
First, the overall trend of innovative drug declaration in the past five years
Since 2015, the CFDA review and approval reform has continued to advance, and a number of highly-respected reform policies have been issued. For example, the “Opinions of the State Council on Reforming the Approval System for Drug Medical Device Evaluation†(Guo Fa [2015] No. 44) has opened a new In the first round of the reform of the drug trial, the State Bureau announced in 2016 the “Notice on Soliciting the Opinions on How to Determine the Principles of Applicants for Priority Reviewâ€, marking the official landing of the drug priority review system. Recently, CFDA has successively published the "Technical Guidelines for Accepting Data on Overseas Clinical Trials of Drugs", and there are reports that it will accelerate the entry of Indian drugs into China.
The effect of the reform is obvious. The backlog of drug review is rapidly being resolved. The process of listing of innovative drugs in China has begun to accelerate significantly. The specific performances are as follows: 1) In 2013-2018H1, the number of new drugs registered in China is increasing year by year; 2) Significant clinical value of the rapid drug listed in China, such as AstraZeneca Oxitinib mesylate, BMS / Aibowei / Gilead and other direct hepatitis C antiviral drugs, cutting-edge biology Aiboweitai, Hengrui Medicine 19K The listing of Merck's nine-price cervical cancer vaccine and BMS Odivo and other heavy-duty varieties has brought more treatment options to Chinese patients. With the deepening of the review and approval reform of the National Bureau, the IND application for the innovative drug and the approval of the listing review will be further accelerated. In the future, more and more innovative drugs will enter China quickly.
  Trends in innovative drug declarations in the past five years (according to the acceptance number)
Remarks: 1) Supplementary application, review and import re-registration are not included in the scope of analysis; 2) Statistical time range is from January 01, 2013 to June 30, 2018; 3) Chemicals Category 1 + 5.1 (Registration Classification 2016 Edition)
Second, the characteristics of 2018H1 innovative drug declaration
According to the data of the Rubik's Cube database, from January 10, 2018 to July 4, 2018, CDE has hosted 338 innovative drug acceptance numbers, including 300 domestic acceptance numbers and 38 import acceptance numbers, involving a total of 197 different varieties.
1. Anti-tumor and immune modulators are the most popular disease areas
Statistics show that anti-tumor and immunomodulator (L class) acceptance number total 170, accounting for 51%, is the most popular reporting field, followed by systemic anti-infectives (J class) 24, central nervous system (N Classes, 21, 16 digestive tracts and metabolism (Class A).
  2018H1 innovative drugs declared in the field of disease distribution
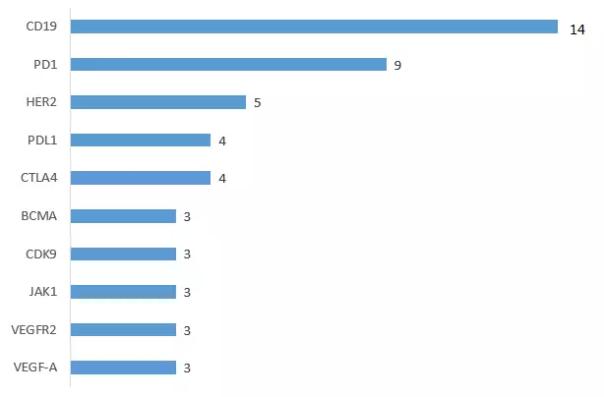
2. CD19 is the hottest target
From late 2017 to 2018, cell therapy declarations began to explode, and cancer immunotherapy products continued to increase worldwide. The registration of Chinese immunotherapy products was also very hot. The data of the drug Rubik's Cube showed that 10 of the 197 varieties declared by 2018H1 The product target is CD19, CD19 becomes the hottest target, followed by PD-1, HER2, PD-L1, CTLA4, BCMA.
  2018H1 innovative drug declaration target heat
Note: Individual drug targets have not been published, not counted
3. Encourage accelerated research and evaluation: 16 priority reviews and 96 special approvals
CFDA encourages innovation and accelerates the process of listing innovative drugs. In 2009 and 2016, CFDA issued the “Regulations on the Special Approval of New Drug Registration†and the “Opinions of the General Administration on Resolving the Approval of Drug Registration Applications for Priority Review and Approval†(Food and Drug Supervision) Guan [2016] No. 19), China's new drug review began to speed up.
According to the data of the Rubik's Cube, among the 197 varieties declared by 2018H1, 16 varieties entered the priority approval list, and 96 varieties were specially approved.
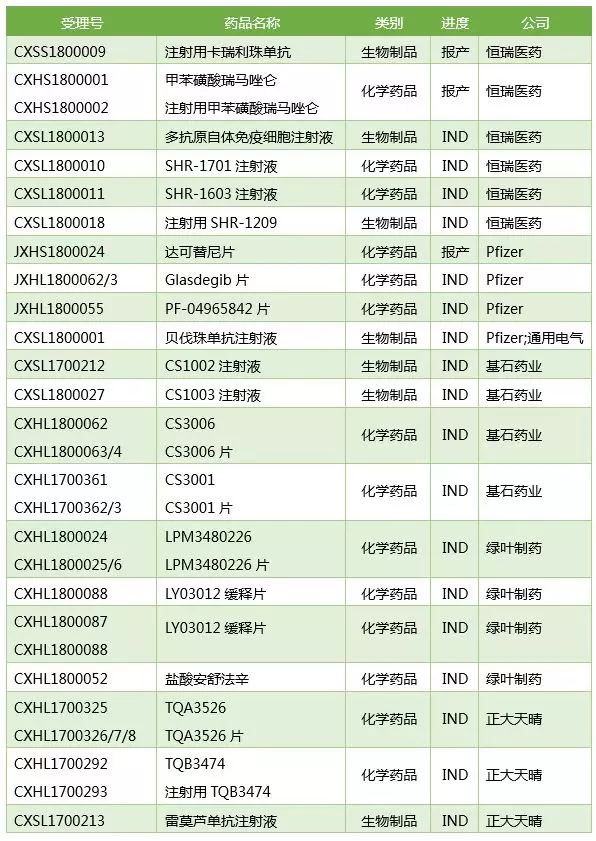
4. Hengrui innovative drug declarations are the most
2018H1 Hengrui has 2 new drugs (NDA, by variety) and 4 INDs, which is the largest number of innovative drugs, followed by Pfizer's 1 NDA and 3 IND, and the cornerstone drug 4 IND. Shandong Green Leaf 4 IND, Zhengda Tianqing 4 IND. The specific varieties are shown in the table below.
Note: Remoruzumab is a biosimilar drug
Third, the focus of new drug application varieties inventory
Among the 338 acceptance numbers declared by 2018H1, there are many hot areas and key varieties worthy of attention, among which:
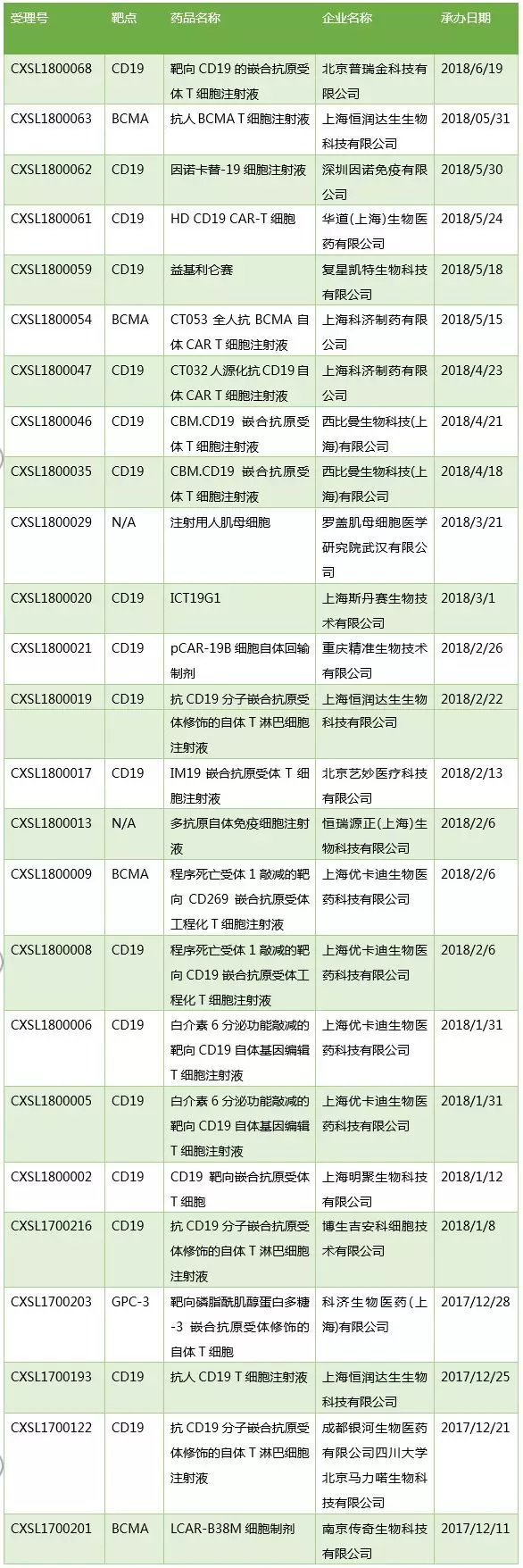
1. Cell therapy products added 21 acceptance numbers
Since December 2017, the application for cell therapy IND has started to increase, and 2018H1 has received 20 new acceptance numbers, and 25 acceptance numbers have been accepted for acceptance. Among them, Nanjing Legend, Mingju Bio (Pharmacological Mingnuo related company) and Hengrun Dasheng's products have been approved for clinical. In addition, the revitalization of Kate CAR-T products Yiji Lilun competition clinical trial application for CDE acceptance, the productization process is worthy of attention.
2. 4 anti-PD-1 monoclonal antibodies were reported
Immunotherapy has brought epoch-making innovative treatments to cancer treatment. Five PD-1/PD-L1 monoclonal antibodies have been approved for marketing worldwide. Up to now, BMS Opdivo has been approved for marketing in China, and Merck Pablo Anti-Shanghai Junshi Teripril Monoclonal Antibody, Cinda Bio-Dili Monoclonal Antibody, Hengrui Medicine Karelizumab is in the technical review stage and will be approved for listing.
3. Hengrui anesthetic drug remamate
Remazol toluene sulfonate, a short-acting GABAa receptor agonist, is another highly regarded anesthetic product of Hengrui Medicine. This product is superior to similar products of remiazolam and remarazol besylate. With higher stability and safety, the product launch will further optimize the Hengrui anesthetic business.
4. The third generation of the EGFR inhibitor evittini maleate
On June 22, 2018, Essen Pharmaceuticals reported that the third-generation EGFR inhibitor, eviatinib maleate, was produced compared to the first generation (gefitinib, ectinib) and the second generation (Affi EGFR inhibitors, ivitinib and other third-generation EGFR inhibitors are still effective in patients with EGFR T790M mutations. The sales of similar products, oxitinib, reached 960 million US dollars in 2017. Ni's sales are expected to be positive.
5. Kain direct anti-virus hepatitis C drug
At present, a number of direct antiviral drugs from five companies including Gloria, Gilead, BMS, Aibowei and Merck are listed in China. Kain KW-136 is an NS5A inhibitor, given direct antiviral drugs. Excellent hepatitis C cure rate, the listing of a variety of drugs will bring new treatment to Chinese patients with hepatitis C.
Disposable Laparoscopic Trocar,Disposable Endoscopic Trocars,Laparoscopic Trocar,Laparoscopic Trocar With Cannula