Li Hong, Director of the Consumer Products Industry Division of the Ministry of Industry and Information Technology: Good morning to all leaders, distinguished guests and leaders. The China Pharmaceutical Enterprise Management Association today held the “6th Presidential (Expanded) Conference of the 6th Party in 2011 to fully summarize the Association 2011 In this year's related work, I will study and discuss the hot and difficult issues in the current development of the pharmaceutical industry, and will play a positive role in the in-depth development of the work of the association. On behalf of the Consumer Products Industry Department of the Ministry of Industry and Information Technology, I would like to extend my warm congratulations on the convening of the conference. The leaders, industry experts, and business representatives expressed their highest respect.
Industry associations are an indispensable part of a mature market economy system and a bridge and link between government and enterprises. Giving full play to the role of industry associations is of great significance for fully implementing the scientific concept of development, accelerating the transformation of government functions, promoting the healthy development of the industry, upgrading the structure, and promoting the path of information industry with Chinese characteristics. Here, I am very happy to take this opportunity to introduce the work of the Ministry of Industry and Information Technology in the formulation of the 12th Five-Year Plan for the pharmaceutical industry.
The “Twelfth Five-Year Plan†of the pharmaceutical industry began with the establishment of a drafting group and a group of experts. It took more than a year for the planning and preparation work. During this process, we did a lot of investigations and research and fully listened to the relevant local departments and trade associations. With the opinions and suggestions of key business circles, the organization of industry associations, research institutions, and experts drafted 14 specialized research reports, which provided favorable support for the formulation of plans. The draft plan was completed at the end of 2010 and has since solicited opinions from various parties and will be issued soon.
Here are some of our main ideas:
First, implement the task of medical reform. To deepen the reform of the pharmaceutical system, it is required to ensure the safe use of drugs by the people and to strengthen the monitoring of the essential drug production industry. We will implement the opinions on deepening the reform of the medical and health system and put forward the overall requirements for promoting the improvement of independent innovation capabilities and optimization and upgrading of the pharmaceutical industry structure of pharmaceutical companies. It is proposed to speed up the development of innovative new drugs for innovative products, strengthen technological transformation of enterprises, raise the level of production technology for large pharmaceutical products, improve the quality and safety of drugs, and achieve the coordinated development of essential drugs and non-essential drugs.
Second, implement relevant tasks for cultivating and developing strategic emerging industries. The state cultivating and developing strategic emerging industries provide a rare opportunity for the development of biopharmaceuticals. In order to gradually realize the goal of nurturing the bio-industry as a pillar industry of the national economy, it plans to formulate the “Decision on Accelerating the Cultivation and Development of Strategic Emerging Industries†and formulating a The strategic emerging industries in China developed the priorities for the development of biopharmaceuticals proposed in the “12th Five-Year Plan†and set up chapters in key areas, and proposed biological basic drugs, new varieties of chemical drugs, modern Chinese medicine, advanced medical devices, packaging materials, pharmaceutical equipment, etc. The focus of product technology development. Focusing on major market demands, it seeks major technological breakthroughs and seizes the commanding heights of science and technology, and plays a leading and leading role in the long-term development of the pharmaceutical industry.
Third, the transformation and upgrading of industries will be driven through continuous innovation. The emphasis of the plan is to speed up the development of new products, combine the implementation of major new drug creation projects, promote the development of major diseases, multiple diseases, and innovative drugs, and at the same time realize the development and production of a number of clinical drugs and large patented new drugs, filling the domestic gaps. The second is to emphasize the transformation of old products. In accordance with international advanced standards, the company will carry out secondary development and reinvention of generic generic drug varieties, striving to be consistent with the original drug in terms of clinical efficacy and safety. Strengthen the technological transformation of enterprises, improve the use of high technology to transform the traditional pharmaceutical industry. The third is to strengthen the establishment of a pharmaceutical innovation system, give full play to the main role of the company in the technological innovation system, build a high-level comprehensive innovation drug research and development platform and unit technology research platform, and improve the pharmaceutical innovation support service system.
Fourth, we will increase industrial concentration as the focus of structural adjustment. One of the planning proposals is to encourage advantageous companies to implement mergers and acquisitions and improve the industrial chain. Improve the efficiency of resource allocation and realize large-scale and intensive operations. The second is to seize the opportunity of the rapid growth of the pharmaceutical market and nurture a group of large-scale enterprise groups that have international competitiveness and have a strong leading role in the development of the industry. Thirdly, strict market access, increasing the technical threshold for the review and approval of drugs, and strictly controlling the number of new start-up pharmaceutical companies. Weed out a batch of enterprises with low production levels and weak management capabilities.
Fifth, the quality and safety of medicines are placed in an important position. Raising the quality of medicines is a matter of people's livelihood and an important symbol of the improvement of the overall quality of the pharmaceutical industry in China. The plan emphasizes that the first is to promote enterprises to improve the quality management system and improve the production environment standards. Strengthen the main responsibility of enterprise quality and establish a sense of corporate integrity. The second is to speed up the quality management and international standards, and encourage qualified companies to carry out GMP certification of developed countries WHO.
Sixth, strengthen the coordination of relevant policies. Due to its particularity, pharmaceutical products are affected by the relevant policies, especially the policies on drug price bidding and procurement, quality supervision, medical insurance, and clinical use of drugs. To achieve various planning goals, it is necessary to strengthen industrial policy guidance. Related policies coordinated and formed a joint force. In the assurance measures, the plan also proposes to improve the relevant policies, including incentives to innovate and standardize competitive drug pricing policies, reflect the quality and priority of the tender procurement policies, and promote the pharmaceutical industry innovation and industrial restructuring of the quality of regulatory policies to promote Rationally use drugs and support innovative medical insurance policies. Efforts will be made to create a policy environment conducive to the restructuring of the pharmaceutical industry.
The above are some of the major considerations in our planning. We hope to work together with all of you here in the future to promote the implementation of planning tasks and the realization of planning goals. Finally, I wish the meeting a complete success. Thank you!
Explosion Proof Solvent Recovery Machine
We occupy a quality position of the Explosion-proof Solvent Recovery Machine in the market place, solvent recovery machine is the layout of the factory, The solvent recovery machine output of our factory is the largest; 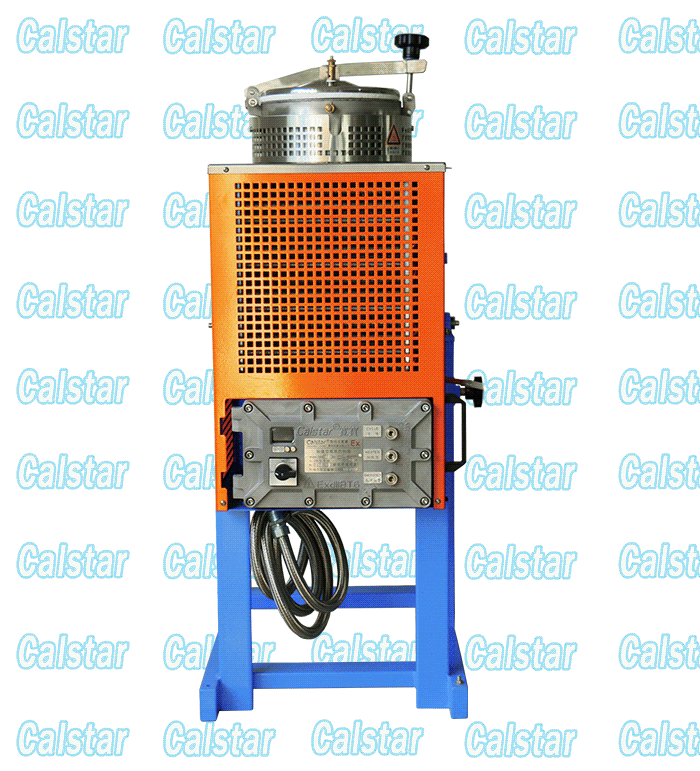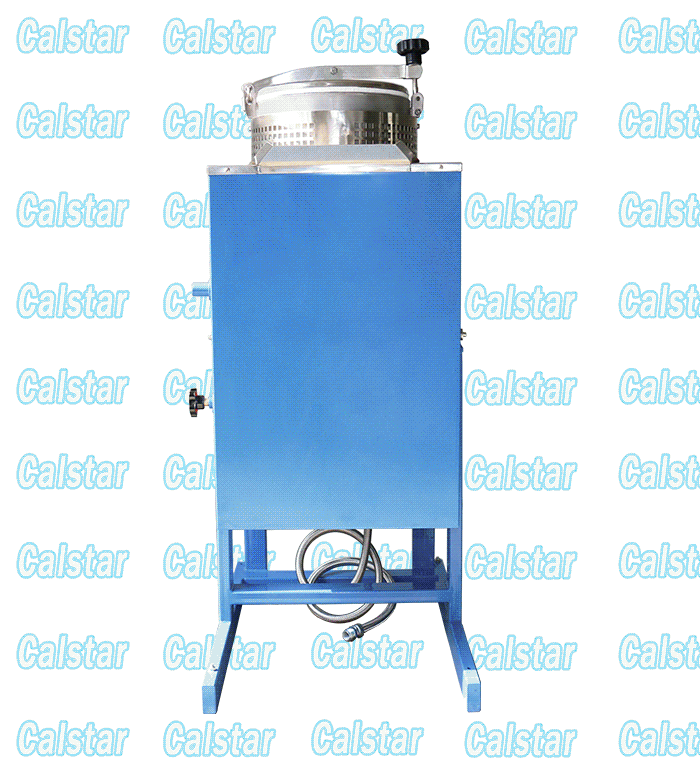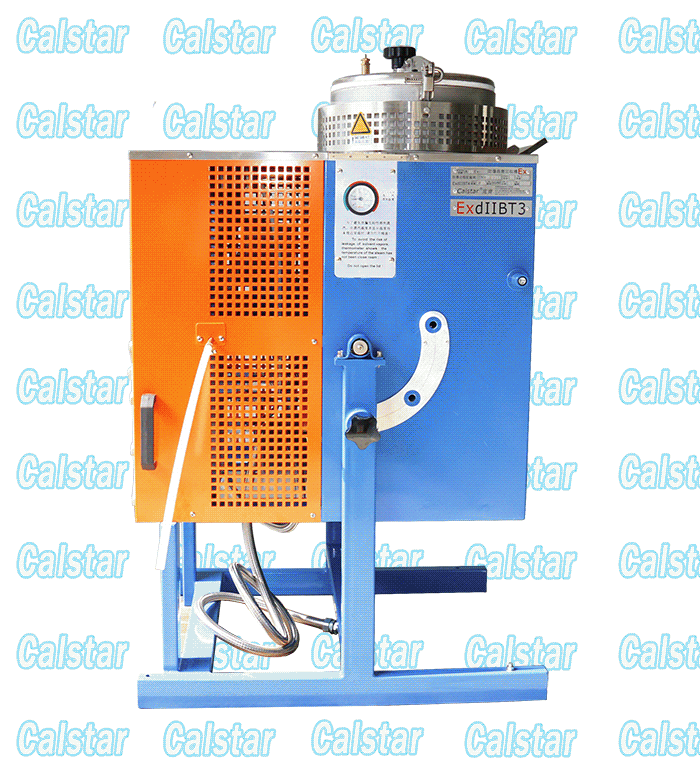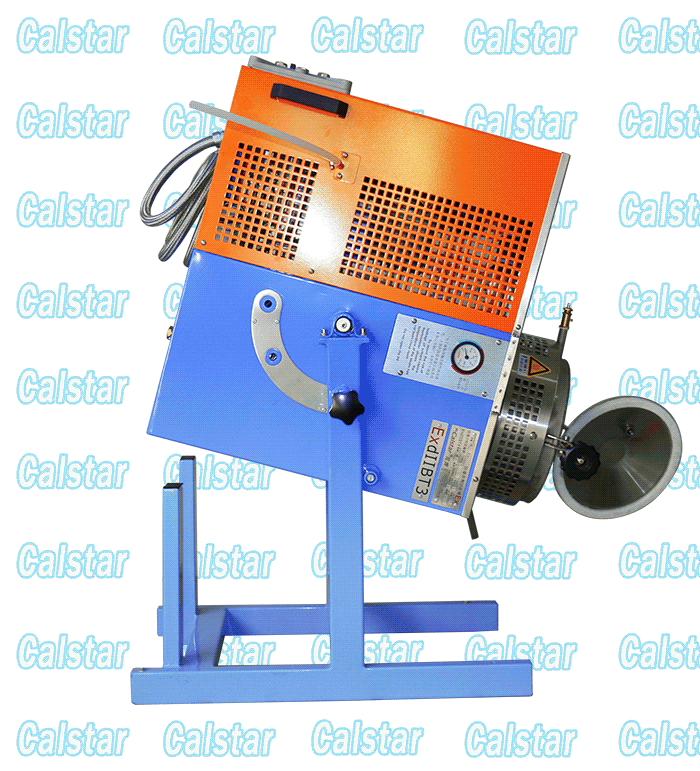
explosion-proof device for recovering solvent, solvent recovery apparatus and other products through the national explosion-proof inspection center Chinese verification, access to safe explosion-proof certification, so Calstar® brand can be assured.
Characteristics of explosion-proof solvent recovery machine
1, the two stage heating setting: according to the composition of the mixed solvent to recycling, the heater temperature for two set, so that different boiling point solvents in accordance with the order from low temperature and high temperature distillation in batches, the start time to avoid too high temperature, resulting in low boiling boiling over solid component and pipeline obstruction by;
2, fan running time setting: after the machine is shut down, the fan can continue to operate for a period of time, in order to improve the safety of operation;
3, the operation is simple: do not need someone to operate, staff only need to feed, turn on the power, clean up the residue can be, the whole process of automatic recycling automatic shutdown; save human resources
4, after the recovery of solvent quality as new: because the device uses the distillation separation principle, do not add any chemical composition
5, model a variety of: your company can choose according to the actual amount of waste of different models of models
6, the cooling way: the use of air-cooled cooling principle to improve the cooling effect, shorten the recovery time, more energy saving and environmental protection,
6, automatic shutdown system: can be controlled by time or temperature
7, recovery tank can be dumped, easy to remove residue
8, double circulation cooling, environmental protection without pollution,
9, fixed temperature shutdown: solvent recovery is completed, according to the steam temperature automatic shutdown;
10, time off: according to the time, double shutdown protection function;
11, ultra high temperature protection: when the machine exceeds the set temperature, automatic stop heating system;
12, temperature display no contact, no electric spark;
13, the temperature is accurate: the digital setting display temperature, the minimum value is 0.1
Explosion Proof Solvent Recovery Machine,Blast Proof Recovery Unit,NMP Solvent Recovery Machine,Solvent Recycling Companies
SHENZHEN KUANBAO ENVIRONMENTAL EQUIPMENT CO., LTD , https://www.calstarkb.com