Sequenom is a representative case of foreign gene sequencing mid-stream companies. The company's core business is non-invasive prenatal genetic testing (NIPT), with 180,000 samples tested in 2015 (the most mainstream product is MaterniT21 for Down's screening). Several other companies in the same field include Verinata Health (Verifi), Ariosa Diagnostics (Harmony) and Natara (Panorama).
From the development of Sequenom, the development of NIPT has three characteristics.
First, non-invasive prenatal testing for high-risk maternal Tang screening in the United States has been widely recognized by commercial and federal government insurance. When Sequenom disclosed its 2015 financial report, it said that 75% of its testing services were paid by insurance. However, as the coverage of insurance payments increased, the amount paid was also rapidly decreasing. In the fourth quarter of 2015, the average price of the insurance payment Sequenom service was $800 per test, while the average payment in 2013 was $1,200. It can be seen that although insurance has included these services in the scope of reimbursement, it has brought a quantity, but as the payer is bound to continue to rely on the advantage of quantity to drive down the price, Sequenom's income has also been declining.
Chart 1: Sequenom's total revenue, loss and sample size changes in the past three years
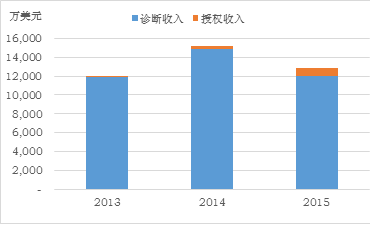
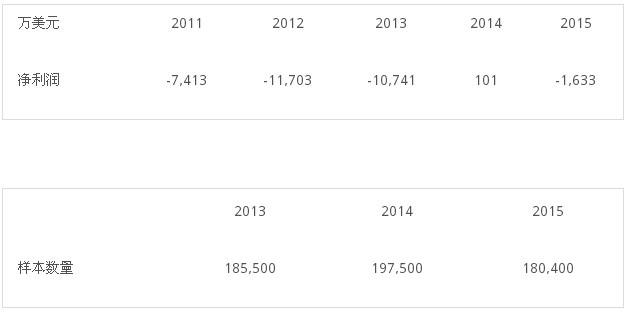
Second, before 2014, Sequenom's revenue came from providing non-invasive prenatal testing directly to medical institutions, most of which was covered by commercial or federal government insurance. But in December 2014, Sequenom and Illumina reached a patent pool agreement that included more than 400 patents related to non-invasive prenatal testing, marking the previous two companies' non-invasive prenatal testing technologies. A settlement was reached on the patent lawsuit, whereby the entire patent pool was jointly owned by Sequenom and Illumina. Since 2015, Sequenom and Illumina have licensed this patent pool to customers for patent fees. This is also the reason why Sequenom's total revenue fell by 15% in 2015 compared to 2014. Some users turned into patent fees and obtained non-invasive prenatal testing after obtaining patent authorization. As a result, Sequenom's direct test revenue fell by 19%. The number of samples sent for inspection also dropped from 197,000 in 2014 to 180,000 in 2015.
PCR ( Polymerase Chain Reaction)
It provides a way to make more copies of a portion of DNA.
We need to have PCR ingredients that include DNA portion, Buffer solution, Primer+DNA (tap polymerase), and Nucleotides during the preparation step.
In the step of PCR sequence, there are three steps in this section.
Step 1 Denaturation
The addition of heat is needed to separate the two strands of the DNA molecule.
Step 2 Annealing
Temperature, is going to be cool in this step, should allow the primers to bind to the specific segments.
Step 3 DNA Synthesis
To make more copies of the DNA.
Those three steps above keep repeating to make enough copies of the DNA fragments. It is the principle of how PCR machine works.
SARS-Cov-2, One test type that uses a sample from a nose or throat swab in a 'PCR test'. The goal is to make enough copies of the viral cDNA in order for it to be detectable.
Superyears General 2215 RT-qPCRadopts all-in-one with an industrial art design, combining the Analytical Instrument, Large touch screen, and Computer. General 2215 RT-qPCR is equipped with the proven thermal-cooled electric technology, which can recognize the speed rate of temperature control faster and obtain high-quality testing results within 40 minutes. To ensure the sensitivity and stability of the testing a design with traditional beam path with a susceptible CMOS image sensor is used. In addition, General 2215 supports multiple PCR analysis and High-resolution Melt Curve (HRM) analysis, compatible with the mainstream reagent kit in the market. It will be the best assistant and your preferred RT-qPCR.
Rt-Pcr For Sars-Cov-2 Testing,Real Time Pcr Machine Kary Mullis,Pcr Machine Covid-19 Nucleic Acid,Qpcr Machine Covid-19 Omicron Detection
Nanjing Superyears Gene Technology Co., Ltd. , https://www.superyearsglobal.com