
GSK is approved, the dawn of gene therapy is coming
The so-called gene therapy is to put a therapeutic gene into the patient's cells, thereby replacing the missing and dysfunctional genes, and finally to achieve a cure for a certain disease. Although the gene therapy program was approved in the United States in 1990, gene therapy was actually approved in the clinic, but it was rare. However, a recent news is undoubtedly a shot in the arm for those who are committed to medical transformation. On May 27th, the gene therapy Strimvelis of the global pharmaceutical giant GlaxoSmithKline (GSK) was approved by the European Union. The approval of GSK represents the formal entry of large pharmaceutical companies into the commercialization of gene therapy, and is another milestone in the market for gene therapy. This area is no longer a mirror, but a new round of rush, will eventually become a bloody hurricane competition.
Of course, Strimvelis is not the world's first approved gene therapy drug. As early as 2012, the European Union has approved the gene therapy drug developed by UniQure for lipoprotein lipase deficiency, Glybera. Glybera encapsulates the functional LPLD gene into AAV (adeno-associated virus) and injects it into the patient's skeletal muscle. Through a one-time treatment, a long-term reduction in the incidence of acute pancreatitis is achieved.

Early gene therapy, because of the uncontrollable immune risk, makes people love and hate. AAV, as the lowest immunogenicity, does not integrate into the nuclear genome, and the best infection vector brings new hope for gene therapy (see The article on June 23, "Medical Black Technology: Treating Diseases Without Medicine, Light is enough"). Glybera fired the first shot of AAV gene therapy and opened up the possibility of gene therapy. Four years have passed, and what remarkable progress has AAV made in the field of gene therapy?
At present, there are more than 100 clinical trials of adeno-associated virus registered with the FDA, most of which are concentrated in clinical phase I and phase II, and one case is completed in the third phase of clinical recruitment. Today we will introduce you to the latest and more influential cases in the first half of 2016.
AAV combined with Crispr/Cas9 gene editing
The conventional AAV technology mainly focuses on the introduction of the outer edge gene into the body, and is expressed safely and stably. As a new gene editing technology, Cas9 is widely considered to have broad clinical application prospects. Its combination with AAV makes genetic modification more targeted and efficient, and the two complement each other.
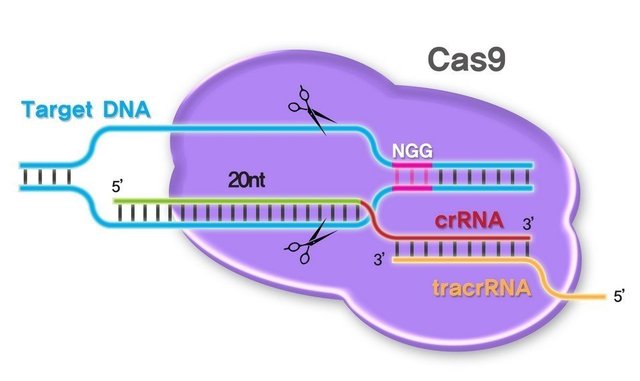
In February 2016, Professor James Wilson of the AAV Carrier Manufacturing Institute of Gene Therapy at the University of Pennsylvania used AAV to treat the rare metabolic urea circulatory system disorder. The disease is caused by a loss of ornithine transcarbamylase (OTC). When an enzyme in the enzyme system is lost or defective, ammonia accumulates in the blood, circulates to the brain, and eventually damages the brain. It is reported that one out of every 40,000 born babies suffer from a loss of OTC. The James Wilson team specifically introduced the nuclease Cas9 as a shearing tool into hepatocytes using the AAV8 serotype vector. Another vector brings gRNA to a specific DNA target site to achieve site-directed mutagenesis. This study effectively improved the survival rate of pathological model mice. The article was published in Nature Biotechnology and has been cited many times since its publication 4 months.

Chinese scientists are not willing to use the combination of CRISPR/Cas9 and AAV. For example, in March 2016, Professor Liu Mingyao and Professor Li Dali, Dean of the School of Life Sciences of East China Normal University, demonstrated for the first time the feasibility of repairing F9 gene mutations to treat hemophilia B by Cas9 gene editing technology. Professor Liu’s team discovered a novel mutation Y371D of the FIX gene for hemophilia B, and found the corresponding amino acid site Y381D in mice, and constructed a C57BL/6 strain of severe B-type hemophils in mice using CRISPR/Cas technology. Disease model and protein sequence 383 displacement code mutation Y383STOP. Then, the AAV-incorporated CRISPR/Cas technology was used to repair the Fix gene in situ. The adeno-associated virus was injected into the tail vein to deliver Cas9, sgRNA and repair templates, and some mouse liver cells were successfully repaired. The blood coagulation ability of F9Y381D hemophilia mice and the survival rate in the tail-tailing experiment were improved. The article was published in EMBO Molecular Medicine with an impact factor as high as 9.547.

AAV breaks through the blood-brain barrier
The blood-brain barrier isolates the brain from the external environment, preventing harmful chemicals and bacteria from invading our brains and protecting the brain, but at the same time about 95% of oral and intravenous drugs are blocked by the blood-brain barrier. This has led doctors to choose to directly inject the drug into the brain during the treatment of neurodegenerative diseases such as Parkinson's, and this invasive method requires drilling on the skull. In a recent study, neuroscientist Viviana Gradinaru used bioengineering to obtain a harmless virus that can cross the barrier, the new recombinant adeno-associated virus AAV-PHP.B, which delivers therapeutic drugs to the brain. This method and virus will become a breakthrough research tool and have a very large clinical application space.

AAV and optogenetics
In January 2016, the team led by Academician Duan Shumin and researcher Wang Hao of Zhejiang University revealed the neural circuit basis of the mouse's congenital fear induced by natural enemy odor. The use of optogenetics, animal behavior and viral reverse tracing techniques found in the mouse brain that the pathway from LHb (nucleus) to LDT (dorsal lateral capping) plays a decisive role in the congenital fear induced by natural enemy odors. The role. Two different subtypes of inhibitory neurons in LDT (PV positive and SOM positive) have completely opposite bidirectional regulation of the fear response. These new findings provide new potential targets for the treatment of fear-induced anxiety and depression. The results were published in Nature Neuroscience with an impact factor of up to 16 points.

From the above several latest articles, we can see that AAV can be said to be the hottest, safest and most likely carrier for clinical application in the field of gene therapy! After ten years of grinding a sword, Frost Blade has never tried; the era of AAV's dominance of gene therapy is really coming!
Thank you for your attention to the AAV small class series! In the next issue, we will discuss the practical operation of AAV and teach you how to improve the experimental level and data quality!

Caladium Praetermissum,Caladium Bicolor,Caladium Humboldtii,Caladium Cultivars
fanhua nursery , https://www.fanhuanursery.com