purpose
Rapid chiral separation of benzyl mandelate and enantiomeric excess at 0.02% impurity level were demonstrated using the Waters ACQUITY UPC2TM system.
background
According to a September 2005 issue of Chemistry and Engineering News, nine of the top 10 pharmaceutical products in the market contain chiral active ingredients, and five of them contain a single enantiomer active ingredient. A single enantiomeric chiral drug is considered to be an improved chemical entity that provides higher pharmacodynamics, better pharmacological data, and more favorable adverse reaction data. For the manufacturer of a single enantiomer drug, the undesired stereoisomer should be equivalent to other organic impurities. The International Coordinating Committee for Human Drug Registration Technology (ICH) has clearly defined the regulatory requirements for the identification, quantification and control of impurities in pharmaceutical substances and their preparations. According to the requirements of ICH, the identification and quantification threshold of organic impurities is 0.1% of the main compound.
The high detection sensitivity of the ACQUITY UPC2 system enables the identification and quantification of enantiomeric impurities in pharmaceutical substances.
solution
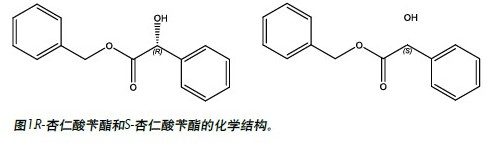
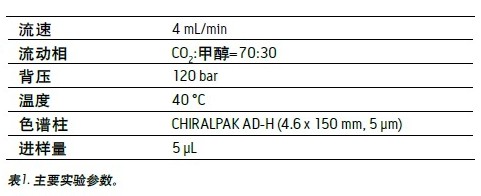
The benzyl mandelate shown in Figure 1 is an important intermediate for the synthesis of pharmaceuticals. A racemic mixture of R- and S-benzyl mandelate (each enantiomer was dissolved in methanol at a concentration of 0.20 mg/mL) using UltraPerformance Convergence ChromatographyTM (UPC 2 TM) for chromatograms. Figure 2 shows. The main experimental parameters are listed in Table 1. The total analysis time is less than 1.5 minutes. The average peak width is less than 6 seconds. The ratio of R- and S-mandelic acid benzyl ester based on the peak area was 0.997.
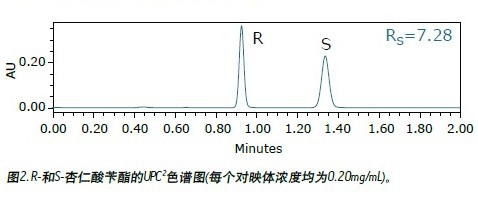
Â
As shown in Table 2, it is the reproducibility data of retention time and peak area for 5 consecutive injections. At a concentration of 0.20 mg/mL, the reproducibility RSD value of the retention time was better than 0.23%, and the peak area reproducibility RSD value was better than 0.5%.
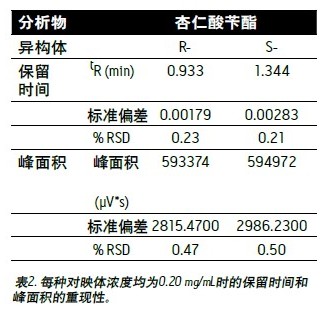
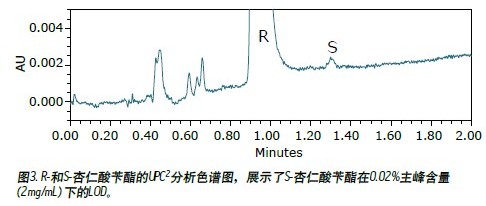
Figure 3 shows the UPC 2 chromatogram of R-mandelic acid benzyl ester at a concentration of 2 mg/mL. It was confirmed by ultraviolet spectroscopy (results not shown) that the small peak at 1.30 min corresponds to S-mandelic acid benzyl ester. The signal-to-noise ratio of the S-mandelic acid impurity peak is about 3 (detection limit), which is equivalent to 0.02% of the main peak according to the peak area. The improved detection sensitivity is due to the overall design of the ACQUITY UPC 2 system, which includes an improved pump system and an optimized detector design. In this example, the enantiomeric excess (ee) value was 99.96%.
to sum up
The UPC 2 chiral separation of R- and S-mandelic acid benzyl esters was successfully completed in less than 1.5 minutes using the ACQUITY UPC 2 system. Excellent reproducibility was achieved at a concentration of 0.20 mg/mL for each enantiomer (reproducibility RSD of retention time was better than 0.23%, peak area RSD was better than 0.5%). The new pump system and detector optimization design results in higher detection sensitivity, making it possible to determine 0.02% enantiomeric impurities and enantiomeric excess. The AQUITY UPC 2 system is suitable for the analysis of trace enantiomer impurities, enantiomeric excess determination and QA/QC analysis.
The reagent bottle uses high-quality polypropylene PP / high-density polyethylene HDPE raw materials, non-biological toxicity, 100,000-level purification workshop production environment, multiple quality system certification, ensuring no DNase / RNASE, no hot gland, no internal toxins, sterilization The plastic bottle is subjected to electron beam irradiation, unpacking, greatly enhances user packaging efficiency.

Reagent Bottle,Plastic Reagent Bottle,Hdpe Reagent Bottles,Narrow Mouth Pp Bottle
Jiangsu iiLO Biotechnology Co., Ltd. , https://www.iilogene.com