Early clinical trial results of new ovarian cancer drugs
March 27, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, ImmunoGen announced that the company's antibody drug conjugate (ADC) mirvetuximab soravtansine is combined with Merck's (MSD) anti-PD-1 therapy pembrolizumab to treat epithelial ovarian resistance to platinum therapy. Positive efficacy and tolerability data were obtained in clinical phase 1b/2 trials of cancer (epithelial ovarian cancer, EOC). This clinical data was released at the annual meeting of the Society of Gynecologic Oncology in New Orleans, March 24-27.

Advanced ovarian cancer is one of the leading causes of cancer death in women, and EOC accounts for 95% of ovarian tumors. The current standard of care for EOC is surgical resection and platinum-based chemotherapy. But the efficacy of this strategy on patients is now saturated. The reason for the high mortality rate in patients with ovarian cancer is that most EOCs are already in advanced stage at the time of diagnosis, and although the tumor is initially very sensitive to platinum therapy, up to 80% of patients will relapse. Tumor resistance to platinum therapy is a major challenge in successfully controlling advanced ovarian cancer.
Mirvetuximab soravtansine is an ADC developed by ImmunoGen. It binds a humanized monoclonal antibody (M9346A) that binds to the alpha folate receptor (FRα) to a cytotoxic DM4 molecule via a disulfide bond. 80-96% of EOC tumors continue to express FRα, whereas FRα is not expressed in normal ovarian epithelium, so it is an attractive therapeutic target. When the ADC binds to FRα, FRα is able to transfer the ADC into the interior of the cell, allowing the cytotoxic molecules carried by the ADC to inhibit mitosis in the tumor cells.
In this clinical Phase 1b/2 trial, called FORWARD II, 14 patients with advanced EOC who had received platinum-based chemotherapy and developed resistance were treated with combination therapy with mirvetuximab soravtansine and pembrolizumab. In the subpopulation of patients with moderate or high levels of FRα, the overall response rate (ORR) was 63%, and the mean progression-free survival (PFS) was 8.6 months.
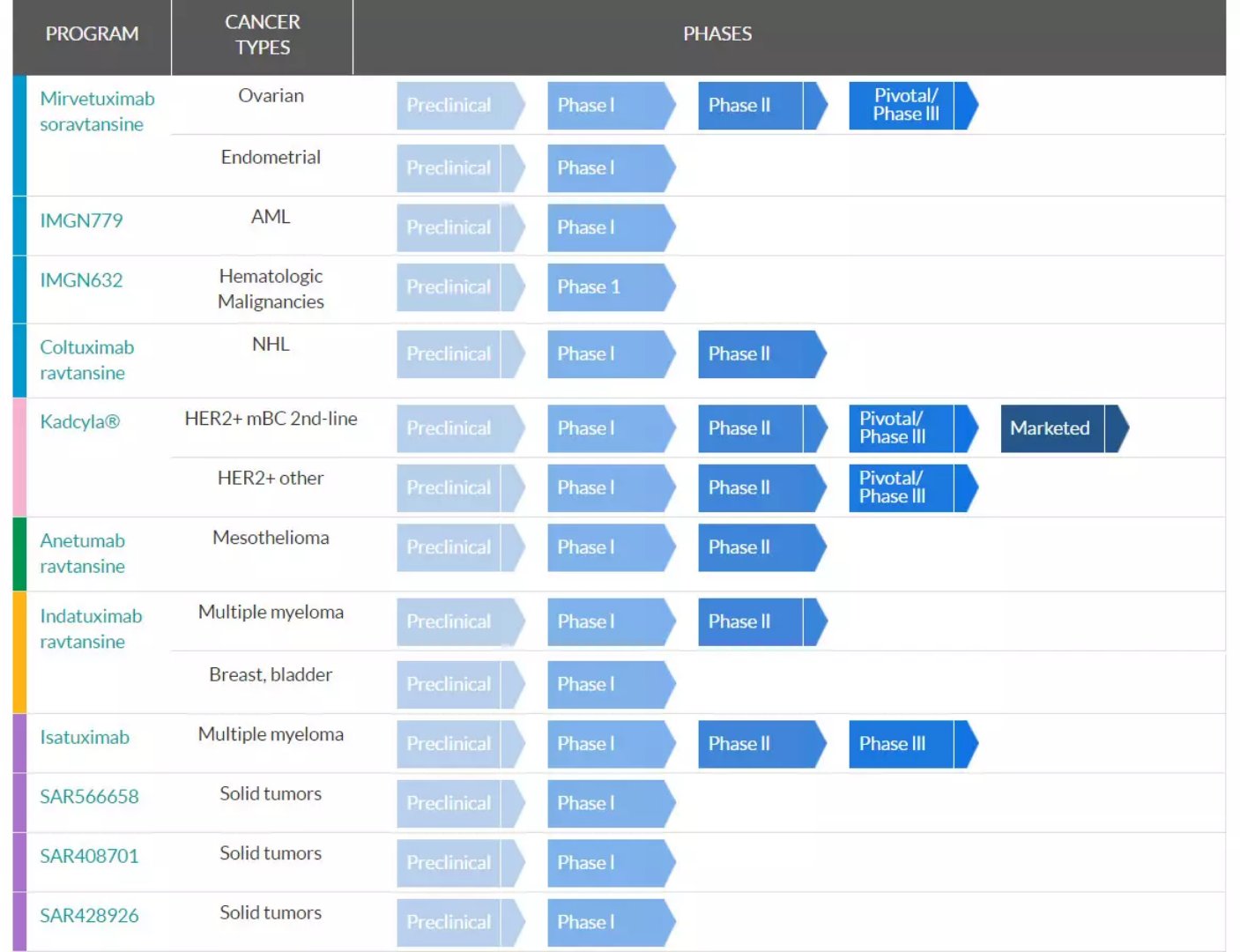
â–²ImmunoGen part of the pipeline (Source: ImmunoGen official website)
All patients included an ORR of 43% and an average PFS of 5.2 months. These patients have received an average of 4.5 other therapies before receiving this treatment, and 64% of them have received treatment with more than 4 different therapies.
At the same time, the test results show that the combination therapy is well tolerated and safe, and the side effects exhibited by the patient are mainly mild or moderate side effects (less than or equal to grade 2).
"We are pleased to see the combination of mirvetuximab and pembrolizumab in anti-tumor efficacy, duration of efficacy and tolerance, especially in patients with moderate or high levels of FRα," we observed the best results, "ImmunoGen Dr. Anna Berkenblit, vice president and chief medical officer of the company, said: "We have demonstrated that the mirvetuximab dose used in our clinical phase 3 single-drug trials can be combined with other therapies in a variety of combinations. These results are consistent. Sexuality shows the potential of mirvetuximab as a member of monotherapy or combination therapy in the treatment of ovaries."
Based on these data, ImmunoGen will additionally register 35 patients with moderate or high levels of FRα to expand this patient population in the FORWARD II clinical trial.
Reference materials:
[1] ImmunoGen Presents Data from FORWARD II Assessment of Mirvetuximab Soravtansine in Combination with Pembrolizumab at the Society of Gynecologic Oncology Annual Meeting
[2] A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer
The Fluorochemicals compound is a new field in modern organic chemistry. It has a special physical and chemical properties, so it is widely used in the earliest development of applied pesticides and rat poison. During World War II it was developed in some countries appliedas antimicrobial agents, there are more and more new research suggests that fluorochemical will play an increasingly more important role in the field of anticancer drugs. We started later in the field of fluorochemicals, but we have a strong sense of his enormous potential, and we are increasing investment to catch up. We will custom product which has potential market prospects from gram and kilogram-class through process optimization, enlarged to the size of ton. Currently we have developed more fluorochemical compounds in the benzoic acid range, the number of products have been more than 2017.
Organic Fluorochemical Compound,FluorineCompounds,Containing Benzoic Acids
Taizhou Volsen Chemical Co., Ltd. , https://www.volsenchem.com