Keytruda treatment HCC clinical data freshly released in 2018 or on an equal footing with Opdivo
January 23, 2018 Source: Sina medicine Author: Dopine
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Merck has announced the latest clinical data of its key product Keytruda clinical trial KEYNOTE-224, which is designed to evaluate the safety and efficacy of Keytruda in patients with advanced hepatocellular carcinoma treated with sorafenib. This article focuses on the latest data from the Keytruda Clinical Phase 2 trial and has compiled key indications and sales data for Keytruda.
1. Clinical Phase 2 trial KEYNOTE-224
At present, Keytruda has been approved for a variety of indications including melanoma, lung cancer, head and neck cancer, etc., and the development trend is very good. KEYNOTE-224 is a one-arm, open-label clinical phase 2 trial designed to evaluate the safety and efficacy of Keytruda in patients with advanced hepatocellular carcinoma treated with sorafenib. The test was summarized.
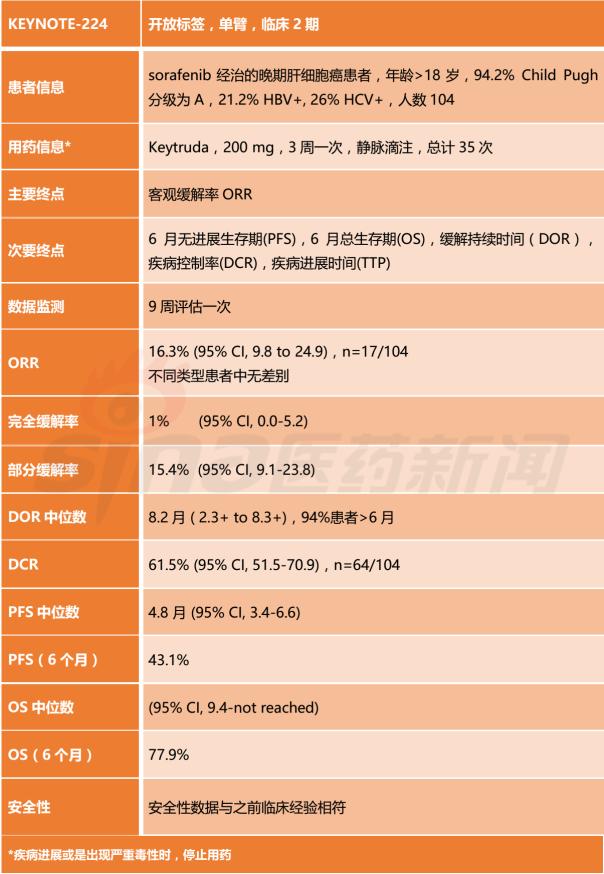
2, Keytruda has been approved for indications and sales data for the past two years
â— Keytruda has been approved for clinical indications
Since the approval of Merck East Keytruda, a number of clinical indications have been seized, notably the approval of unremovable or metastatic solid tumor indications with high microsatellite instability or mismatch repair defects (MSI-H/dMMR). The approval of this indication is a memorable milestone for Keytruda. The following table summarizes the current clinical indications for Keytruda and its approved time:

â— Keytruda will be on an equal footing with Opdivo in 2018
Although Merck and Bristol-Myers Squibb have not yet announced their fourth-quarter earnings for 2017, market data for Keytruda and Opdivo's two heavyweight products are merging, and the gap between Keytruda and Opdivo is shrinking sharply in 2017. It can be predicted that in 2018, Keytruda will be on an equal footing with Opdivo and become the “double arrogance†of the PD-(L)1 market.

Source: Ned Pagliarulo / BioPharma Dive
At present, the four clinical phase 2 and 9 clinical phase 3 projects related to Keytruda in the official website of Merck are progressing, and nearly 400 clinical trials of Keytruda single-agent/combination therapy are underway. Optivo of Bristol-Myers Squibb has obtained the label of advanced hepatocellular carcinoma treated by sorafenib, and it is only a matter of time before keytruda obtains the indication.
Reference Source: First-Time Data for Merck's KEYTRUDA® (pembrolizumab) in Patients with Previously Treated Advanced Hepatocellular Carcinoma (HCC) to be Presented at 2018 ASCO GI Symposium
Freeze Dried Products, Freeze Dried Vegetables, Frozen Dried Food, Premium Freeze Dried Green Vegetables, FD Vegetables
Jiangsu Tiankang Food Co., Ltd. , https://www.tiankangfood.com