Dynamic Imaging of Molecular Assemblies in Live Cells Based on Nanoparticle Plasmon Resonance Coupling
Dynamic image of molecular assembly in cells based on nanoparticle plasmon resonance coupling
Jesse Aaron, Kort Travis, Nathan Harrison, and Konstantin Sokolov
Department of Biomedical Engineering, Department of Physics, UniVersity of Texas at Austin, Austin, Texas 78712, and Department of Imaging Physics, UT MD Anderson Cancer Center, Houston, Texas 77030
Received June 8, 2009; Revised Manuscript Received July 21, 2009
ABSTRACT summary
We used molecular-specific gold nanoparticles to monitor epidermal growth factor receptors (EGFR) in live A431 cells over time. Dark-field hyperspectral imaging, electron microscopy, and electrodynamic modeling were used to correlate optical properties of EGFR-bound plasmonic nanoparticles with receptor regulation We showed that receptor trafficking resulted in a progressive red shift of greater than 100 nm in the nanoparticle plasmon resonance wavelength over a time period of 60 min. further, we demonstrate that changes in peak scattering wavelengths of gold nanoparticles from 546±15 to 574± 20, and to 597±44 nm are associated with EGFR trafficking from the cell membrane, to early endosomes, and to late endosomes/multivesicular bodies, respectively. Finally, we used the changes in scattering spectra of EGFR -bound nanoparticles and a Straightforward statistical analysis of RGB-channel color images of labeled cells to create near real-time maps of EGFR regulatory states In living cells.
In A431 live cells we monitored epidermal growth factor receptor (EGFR) with molecules containing specific gold nanoparticles. The optical properties of EGFR bound to the plasmonic nanoparticles are correlated with the regulation state of the receptor by means of dark field hyperspectral imaging, electron microscopy, and electrodynamic simulation. Under the condition that the nanoparticle plasmon resonance is more than 60 minutes, we show that the transport of the receptor results in a red shift of more than 100 nm. Furthermore, we demonstrate that changes in the peak scattering wavelength of gold nanoparticles (from 546 ± 15 to 574 ± 20 to 597 ± 44 nm) are associated with transport of EGFR (from cell membrane to early endosome to late endosome, respectively) / multi-foam). Finally, using a visual data analysis of the scatter spectra of EGFR bound to nanoparticles and the three primary color images of the labeled cells, we produced images of near real-time EGFR regulatory states in living cells.
The key task of biology is to detect and monitor the interactions between large numbers of biomolecules in cells, because these interactions largely control the behavior of almost all cell types. Imaging is an essential method for measuring the spatiotemporal characteristics of intact cellular protein assembly. In this work, we will develop the application of nanoparticle plasmon resonance coupling (NPRC) and demonstrate a novel and ubiquitous approach by which we can describe cells in nanoscale measurements in living cells. Assemble and image it.
In the past few decades, the application of fluorescence resonance energy transfer (FRET) has enabled many researchers to elucidate the link between important cellular functions in submicroscopic, non-invasive conditions. Recently, image microscopy (ICM) and its variants have been widely used to describe the assembly and clustering of large protein molecules in situ, including EGFR. While all of these techniques are very useful, the FRET technique is only suitable for detecting two very close (?5 nm) single molecules 1,4 of different types. A variety of ICM techniques can be used to measure the relationship and clustering of many molecules at submicroscopic metrology levels, while FRET additional information is simply a statistical average that does not directly reveal the cluster's distribution size or any millimeter-level formation with clusters. Additional information about biomolecules. Moreover, ICM technology is highly sensitive to background interference, and the resolution of image systems is often not fully utilized. In addition, both FRET and ICM technologies are severely limited by photobleaching. These limitations can be avoided in electron microscopy, especially in the case of nanoscale immunogold methods for imaging biomolecule assembly5. However, there are some costs to achieving this requirement, lack of real-time, live cell performance, and the need for a tedious sample preparation process. For all of the above reasons, the use of high-level stereo-analytical methods to quantify cell clusters in living cells requires new physical solutions to develop and complement existing available technologies.
Recently, plasmon resonance nanoparticles have been developed as specific molecular probes for high sensitivity detection of 6-16 and cell photothermal damage 17-20. Compared to the observation of a single nanoparticle, when the two plasmon nanoparticles are very close together, they show the nanoparticle plasmon resonance coupling effect (NPRC), which is represented by a spectrum on the optical cross section of the metal nanoparticle assembly. Drift 21, 22. Spectral drift is largely dependent on the distance between particles, and sensitivity can be easily adjusted by changing the size of the particles. For particle swarms with a center-to-center spacing of less than 3 times the particle radius, NPRC is very important, providing an effective range of detectable interaction distances for tens of thousands of nanometers. Because of this effect, NPRC was used to demonstrate an attractive FRET simulation as a valid proof of the two-state interaction of DNA-DNA, 24-26 DNA-protein, 27 and protein-protein. These studies show that NPRC is not affected by photobleaching and that its extended detection range is not on the same order of magnitude compared to FRET.
We have previously demonstrated that NPRC provides a powerful method for cancer diagnosis by more convenient detection of growth factors that accumulate in cancerous tissues6,29. Now, we have expanded the scope of application of NPRC to study the mechanisms of aggregation and regulation of molecules in living cells. We have developed a new computational framework to obtain information from NPRC spectra, in addition to using a practical image analysis method to infer growth factor receptor regulation.
We observed the trade mechanism of epidermal growth factor receptor (EGFR), a tyrosine kinase (RTK) receptor that controls some of the most basic cellular processes, including DNA replication and cell division. EGFR is also an important tumor marker in therapeutic approaches and has profound implications for the development of new cancer therapies31,32. The intrinsic process of EGFR is a key regulation that determines the behavior of cells. It is well known that membranes bound to EGFR can cause ligand binding to promote signal growth. However, it has recently been found that even after early intracellular EGFR internalization, the signal can continue to be released, eventually stopping 31 after lysosome entry. Both the recycling of EGFR and the process of nuclear transfer have increased the complexity of EGFR30,33. Therefore, the key to the problem is obvious. Effective EGFR involves not only the detection of proteins, but also the nanoscale details of tissue, polymerization and separation in local regions of cells. In this study, we demonstrate that NPRC is a convenient method for searching color image information of live cells with EGFR-specific gold nanoparticles in dark field conditions.
Synthesis of specific gold nanoparticle molecules
Near-spherical gold nanoparticles (average diameter 25 nm) were synthesized using the method described by Frens, and the chloroauric acid 34 was reduced by the chemical method of sodium citrate. By varying the ratio of Au3 + to citrate, the size of the resulting nanoparticles can be controlled. The size of the particles in this work, the concentration of the particles of the proposed gold colloid is about 6 × 1011 particles per ml.
Monoclonal antibodies are bound to the surface of gold nanoparticles using a heterobifunctional link, see 35. Briefly, anti-EGFR monoclonal antibodies (clone 29.1.1 sigma) were removed from ascites using a 100 kDa MWCO centrifugal filter and a HEPSES solution (pH 7.5) at a concentration of 1 mg/mL at 40 mM. In suspension treatment. Add sodium periodate (NaIO4) to the antibody solution and finally store in a weak wind, protected from light for 30 minutes. The concentration is 30 minutes in the last 10 mm. Sodium periodate oxidizes the hydroxyl group located in the glycosylation region of the antibody to an aldehyde group. Then, the solution was diluted 50-fold with 1 × PBS, and then a 200 μM shaped bifunctional connector (Sensopath Technology Co., Ltd.) was added.
This connector consists of a PEG chain and a hydrazide group containing two sulfhydril groups at one end. This newly synthesized aldehyde is concentrated on the connector with the hydrazide group and the residual thiol can be used on the bound gold surface. Excess unreacted connectors were removed by centrifugation as described above, and the antibody-linker solution was stored in 1 mg/mL, 1 x PBS for 4 weeks. Prior to this, to label the cells, the antibody connector solution was first diluted 100-fold in 40 mm HEPES, equivalent to the addition of an equal volume of 25 nm gold nanoparticles. The two parts were mixed gently and stirred for 20 minutes, followed by a 5 kDa monofunctional thiolate polyethylene glycol (mPEG-SH, Nektar) to a final concentration of 10-6 M. Five minutes after the addition of the mPEG thiol, the antibody nanoparticle connector was decelerated for 3 minutes under conditions of 3400 x g, and then the supernatant was removed. The connector was resuspended in a phenolphthalein-free cell culture medium containing 10% FBS.
The use of 25 nm diameter gold particles is due to the high contrast ratio observed when detecting EGFR-expressing A431 cells29. The antibody-bound particles do not block the receptor response by EGF36. Our previous studies have demonstrated that neither monofunctional thiolate polyethylene glycol (mPEG-SH) nor non-specific antibodies bind to nano-ions will affect EGFR-expressing cells. The conjugated particle polyethylene glycol (mPEG-SH) or non-specific antibody with a monofunctional thiolate does not express cells with the epidermal growth factor receptor. Moreover, the substitution and competition phenomena detected under the condition of non-resistant EGFR antibody showed the characteristics of EGFR bound to the gold linker29.
Dynamic image of living cells.
A431 keratinocytes were cultured in a 22 mm square box, and the F-12 (50 / 50) cell culture medium was cultured at 37 ° C, 5% CO2 supplemented with 5% FBS. For live imaging experiments, A431 cells were implanted into optical imaging flow chambers (ibidi μslide chambers, Integrated BioDiagnostics) and allowed to attach overnight. The chamber is then split into two chambers - the chamber and the control chamber. The cells in the cavity were exposed to 10 μM AG1478 (a new phosphorylation inhibitor of Calbiochem), and the cells in the control chamber were not exposed to the inhibitor. The samples were then stored under serum starvation for 24 hours. Place the chamber under the microscope, connect to a peristaltic pump and supply DMEM (with 10% FBS) media at low flow rates (usually 1 mL/min) or supply pure DMEM gold nanolinkers. Use a built-in resistance heater to control the temperature and raise the temperature to 37 degrees before introducing the chamber.
During the time-lapse image, the sample was continuously exposed for 45-60 minutes in the connector at a low flow rate of approximately 1012 particles/mL. The Leica DM6000 upright microscope image was supplemented with a 100 watt halogen source and an oil immersion dark field lens (Leica) with a numerical aperture of 1.2. A long working distance (1.2 mm), 0.75 NA, 63 times objective lens (Leica) was used to acquire the scattered signal, and a highly sensitive 12-byte CCD camera (SPOT Pursuit XS, Diagnostic Instruments) was used to record the image. . To ensure cell viability throughout the live imaging experiment, we performed a calcein AM assay (molecular probe) 2 hours after the cells were exposed to anti-EGFR nanoparticle conjugates. The results show that there is no statistically significant change in the robustness of gold nanoparticle-labeled cells to uncalibrated cells.
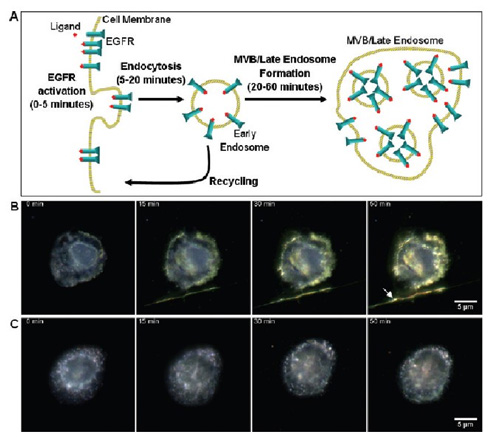
Figure 1 Live cell dynamic image. (A) EGFR transaction map bound according to the ligand. This process was monitored in real time under a microscope in calibrating live cells against EGFR gold nanoparticles. (B) live A431 cells and (C) viable cells pretreated with 10 μM AG1478 (reduced endocytosis). From left to right, the image shows the change in color and intensity of the gold nanoparticle optical signal from initial exposure to nanoparticles to 0, 15, 30 and 50 minutes, respectively. The color of the untreated dark field image varies from blue to yellow orange, which is not visible to the treated cells. The rightmost arrow in Figure B shows the filaments emitted from adjacent cells. Image acquisition requires the use of a dark field illumination, long working distance, 63x, 0.75 NA objective.
Dynamic live cell images of anti-EGFR nanoparticles were shown to show continuous color changes, from green to yellow, and finally to orange (Figure 1B). These color changes are synchronized with known EGFR trading dynamics. Initially, the dimerization and polymerization of EGFR was in the plasma membrane, followed by endocytosis into an early intracellular body. These early endosomes can be recycled to the cell surface or continue to travel within 20-60 minutes to form late intracellular bodies or MVB. AG1478, a potent EGFR inhibitor, will interrupt the effect of continuous color change as it interferes with phosphatidylation and internalization of EGFR (Fig. 1C). These data indicate that the measured color change is related to EFGR intracellular recombination, is caused by ligand binding, and is also due to intracellular vesicle trafficking. In the image of the untreated sample, the relative intensity of red is 34% from 27% (Fig. 1B). Conversely, the increase in the value of the sample treated with the AG1478 inhibitor is only 28% to 30% (Fig. 1C). ).
The cells are calibrated at different temperatures.
In order to establish a relationship between the scattering reaction of the isotonic nanoparticles and the kinetics of the EGFR mechanism in living cells, I calibrated at 4, 25 and 37 °C, respectively. Temperature control allows EGFR activation and trading mechanisms to stop at critical points. The EGFR internalization of cells was inhibited at ?C. Internalization of 25 °C cells forms early endosomes, and finally complete EGFR regulatory mechanisms are obtained by forming multivesicular and late endosomes within 1 hour at 37 °C. 33, 38, 39. For temperature-based experiments, cells were implanted with coverslips at 50 000-100 000/mL and allowed to attach overnight. The cells were then washed with 1× PBS and supplemental medium supplemented with 5% FBS gold nanoparticle biolinker, and then the coverslips containing the nanoparticle cell samples were placed under 4, 25, and 37 °C conditions. Its effect is 60 minutes. The nanoparticles were then aspirated from the sample, then the cells were washed in 1 x PBS and fixed with fresh 4% formaldehyde at 4 °C for 15 minutes. The color image of the cells calibrated at three different temperatures shows a significant change in the scatter signal, from green (4 ? C, Figure 2a) to yellow (25 ? C, Figure 2b) to orange (37 ? C, Figure 2c) ).
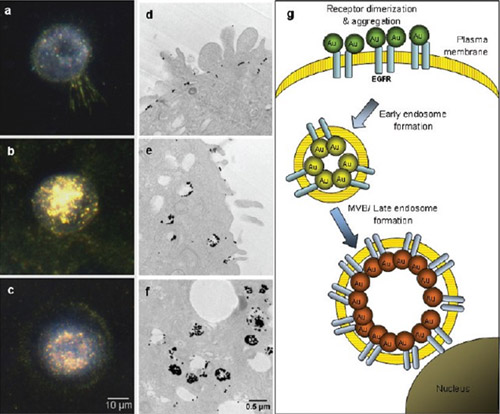
Figure 2 calibrates cells at different temperatures. The dark-field image of A431 cells calibrated with 25 nm anti-EGFR gold nanoparticle conjugates at 4 (a), 25 (b) and 37 ° C (c) is shown. Dark field microscopy reveals light scattering from the sample. The normal EGFR regulatory program is prevented by controlling the temperature. At 4 °C, the receptor stayed on the surface of the cell membrane, and intracellular internalization and multivesicular sorting were performed at 25 and 37 °C, respectively. (d, 4 ?C; e, 25 ?C; and f, 37 ?C) The TEM of the identical sample shows a new arrangement of nanoscale EGFR, which corresponds to the optical image. Figure (g) shows the qualitative relationship between the regulatory phase of EGFR and the optical signals of gold nanoparticles.
Transmission electron microscopy images of the calibrated cells (Fig. 2d-f) show temperature-dependent changes in the regulation of nanoscale EGFR, which was reported to be 39. At 4 ° C, the nanoparticles are placed on the cell membrane of a small cluster. Increasing the temperature to 25 °C results in a more three-dimensional, fuller binding pattern associated with uptake of intracellular bodies. At 37 °C, the EGFR trading process led to a very complex aggregate structure consistent with MVB.
We used hyperspectral imaging to quantify nanoparticle scattering changes associated with EGFR behavior (Fig. 3, ai). Hyperspectral imaging requires the use of the PARIS system (Lightform). Images of the scattered peaks within 530-550 nm were collected at 4 °C (Fig. 3d): under these conditions, EGFR was clearly located on the cytoplasmic membrane. At 25 °C, red drift began to increase as EGFR was mainly concentrated in early endosomes (Fig. 3e). However, at 37 °C, the peak position of the broad spectrum band is obvious (Fig. 3f). At 4 and 25 °C (Fig. 3g and h), the mean and standard deviation of the peak distribution are 546 ± 15 and 574 ± 20 nm, respectively. Interestingly, the peak of the scattering spectrum at 37 ° C (Fig. 3i) is largely offset (mean and standard deviation is 597 ± 44 nm); this is consistent with the multiple mechanisms and trading phases of EGFR in cells. .
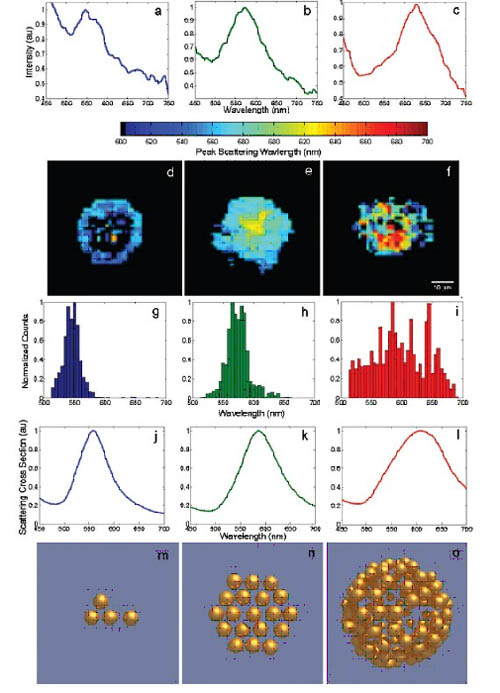
Figure 3. Quantitative relationship between the scattering of bound gold nanoparticles EGFR and the EGFR regulatory phase. Dark field hyperspectral microscopy of cells was calibrated at 4 ° C (left, a, d, g), 25 ° C (middle, b, e, h), and 37 ° C (right, c, f, i). The data was acquired using the microscopic device described in Figure 1. A representative single-pixel spectrum in the cell image shows peaks scattered at approximately 546 (a), 576 (b), and 601 nm (c). The (df) image is a color calibrated according to the position of the scattering peak of each pixel in the field of view. If a pixel has no identifiable peaks corresponding to the spectrum, no color is assigned. (gi) shows the distribution of the peak scattering wavelength in (df). (jl) The following three typical structures are electric simulations of nanoparticle polymer spectra: a 4-particle chain structure (j, m), a 19-particle disk structure (k, n), a 130- The package structure of the particles (l, o). All scattering cross sections are marked for each structure and are accompanied by the corresponding particle distribution details (mo). The effect of the increase in the number of effective particles and the morphological changes from two to three dimensions resulted in significant red drift and broadening of the scattering spectrum.
Metal nanoparticle polymer light scattering electric model
In order to further explore the relationship between the nanoparticle polymer morphology and the combined optical cross section, I implemented a detailed electric simulation. The electric code used to calculate the nano-polymerization simulation is a complete application of some of the new T-matrix code based on C++, which has been developed over the past few years. A mixed multi-threaded Information Channel Profile (MPI) working model is used throughout, using the best modern group computing resources. The specific T-matrix framework extended to polymers is based primarily on the methods explored by Mackowski, Mishchenko 41-43 and Stout44. The details of the dielectric constant function are stated in Aaron et al. In all experiments the incident light was unpolarized and the wave vector pointed to the page as in Figure 3m-o. The gold sphere is 25 nm in diameter and is surrounded by water with a center-to-particle spacing of 2.4 times the particle radius. The fractional standard deviation of the 3D ideal lattice point distribution of the particles is 0.07. Each of the polymerization forms was constructed using the number of polymer particles described in the TEM image, the particle space, and typical values ​​of the entire morphology.
The optical reaction of nanoparticle polymers is complex and nonlinearly dependent on the morphological details of the polymer, and greatly increases the variation in the number of clusters of optical cross-sections. The morphological details of the polymer can show the number, spatial and overall spatial distribution of the acceptor/nanoparticle (such as a two-dimensional plane or three-dimensional structure). Usually the effect of the coupling between particles produces a red drift and a broadening of the resonant peak. Figure 3 (jl) shows the scattering cross sections of three typical structures (Figure 3m-o), which are designed to approximate polymer morphology, Figures 2d-f. Note that the simulated spectrum shows overall redshift and peak broadening, consistent with those depicted in Figures 3a-c.
For random polymers, the average position wave of the particle position has a significant effect on the spectral drift due to the high nonlinear dependence of the isotropic particle resonance coupling in the particle space. Moreover, three-dimensional volume-fillings like those seen in intracellular bodies experience more spectral drift than two-dimensional polymers. This is noteworthy because it shows that NPRC can detect differences in morphology that are related to internalization, which is caused by changes in the corresponding nanoscale EGFR tissue.
Monitor EGFR transactions
Now that we have established a relationship between the EGFR scattering properties of bound nanoparticles and the regulatory phase of EGFR, we examined that the three primary color image levels can also be effectively used to monitor EGFR transactions. The relative intensity of the red band of live cell color images has increased (Fig. 1, B) as a clear indicator of EGFR activation and endocytosis. Therefore, I used this parameter to relate the color change of live cell images to the control phase of EGFR. To achieve this goal, the hyperspectral data provided in Figure 3 is divided into three categories that correspond to the regulatory phases of the associated EGFR, respectively. (1) epidermal receptors, (2) early endosomes, and (3) late endosomes/MVB. At least 5 intact cell aliquots should be analyzed in each group, each representing at least 1000 spectra. Using the resulting spectrum, a statistically significant regional distribution was obtained for each EGFR region normalized red band intensity value (Figure 4). Then for each time-lapse image with no background pixels, we analyze the probability that the standard red band value belongs to each of the relevant EGFR stages. These pairwise comparisons result in three assumed values ​​that are used as a weighting factor when false colors are set for each image of the living cell image. This analysis can form an image in which the color represents the statistical probability of the EGFR control phase (Figure 5).
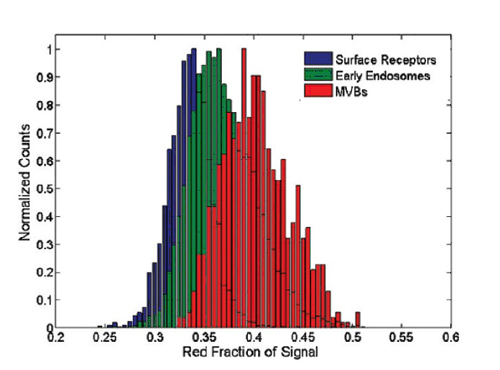
Figure 4. Correlation of red band intensity distribution of EGFR molecules labeled with gold nanoparticles - cell surface (blue), early endosome (green), late endosome (red)
Due to the use of the CCD, the red band collects signals in the 600-700 nm wavelength range. An increase in plasmon resonance coupling in each case results in an overall increase in the intensity of the red band. A pixel analysis of the live cell image of Figure 1 was performed using the pixel principle and compared to the three distributions displayed by a data z-test.
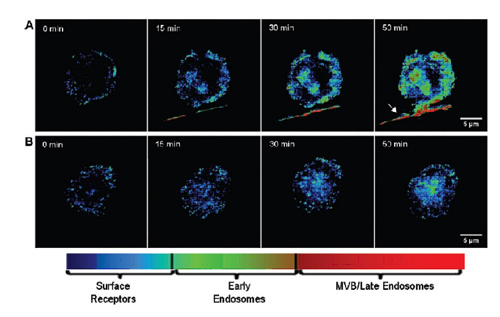
Figure 5 shows the pseudo-color image of live cells distributed in the EGFR regulatory phase. The color-statistic analysis of the time-lapse image shown in Figure 1 determines the color to be assigned to the EGFR distribution—untreated cells (A), cells treated with 10 μM AG1478 (B ). Blue indicates that EGFR is highly likely to be located in the cytoplasmic membrane, green is located in the early endosomes, and red is located in the late endosomes/MVB. Note that AG1478 treatment significantly reduced the endocytosis of EGFR. The rightmost arrow in panel A indicates that the filaments are from neighboring cells, showing a large number of EGFR transactions around their axis.
The use of isotonic ion nanoparticles provides additional information adjacent to nanoscale biomolecular tissue as compared to previous fluorescently labeled studies (e.g., quantum dots 46). It has been demonstrated that optical variations corresponding to particle spacing can be accurately quantified, and that a similar quantitative model for nanoparticle connectors is underway 40 work is underway.
The application of NPRC described herein can be extended to other RTKs and G-protein coupled receptors 33 involved in signal conversion. Systems of other regulatory molecules contain information on the immunological synaptic shape of the integrin cluster. If the interacting molecules are calibrated to show plasmonic nanoparticles with distinct optical characteristics, this method can be used to detect interactions of heterosexual molecules, such as detecting interactions between different RTKs. The theoretical simulations here can be used to optimize the design of nanoparticles that will produce significant optical changes in the case of a single heterosexual interaction.
The main advantage of NPRC as a biosensing tool is the complex optical response associated with plasma nanoparticle assembly. Observations have shown that changes in optical characterization data associated with nanoscale changes in tissues contribute to the development of statistical linkages in biological processes. Further understanding of NPRC is very important for its development as a quantitative biosensing tool. The inhomogeneity of particle shape and the subtle effects of nanoparticle polymers on spectral features (such as scattering peak width) are still under investigation and are expected to reveal a wealth of useful additional information.
NPRC can be used as an independent method or integrated into previously used biophysical methods (eg FRET, 1, 4 ICM, 2, 3 or EM5) to provide additional complete information. TEMs with immunogold labels have been used to study high resolution three-dimensional protein structures in fixed samples. The method used here is to extend the TEM image to live optical dynamic optical images, which are clear information about molecular assembly (for adjacent and nanoscale biomolecular tissues). However, for those methods that do not require super-resolution, NPRC can provide the ability to describe the auxiliary diffraction features, with a range of distances in excess of tens of thousands of nanometers, which clearly exceeds the effective distance of FRET. In addition, NPRC exhibits sensitivity to the geometrical features of the protein family, such as planar two-dimensional and three-dimensional three-dimensional polymers in live cell images, and can track the vitality of the family in real time.
The sensitivity of NPRC to individual clusters at the nanoscale can provide potentially very valuable supplemental population population measurements, such as the ICM method can provide population density and the average number of receptors per population. Moreover, the nanoparticles themselves can also be excellent probes for ICMs because their brightness and lightfastness can promote direct integration of these techniques. We expect NPRC to continue to attract the interest of bio- and biomedical researchers who have a unique insight into the role of biomolecules.
Sterile Vials are produced by aluminum caps, non-latex butyl stoppers and SCHOTT Neutral Type I glass vials. They are approved by cGMP and FDA with internally sterile.The production process is carried out under strict Class 100 workshop. Finished vials can meet the FDA`s authorised 14-day sterility test. Sterile vials are primarily used for mixing different medications or solutions for injection or research applications like HCG, heparin, lidocaine, diabetic medications and morphine for intravenous or syringe injections. Some drugs may need to be diluted or mixed with sterile water or other drugs. Sterile vials are also used for nuclear medicine, PET-CT, Liquid collection.
Sterile Vials
Sterile Vials,Sterile Glass Vials,Sealed Sterile Vials,Sterile Empty Vials
China Lemon Trading Co.,Ltd , https://www.lemonvial.com