Lilly announces the latest research and development pipeline, and many new drugs attract attention
January 15, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Lilly Ricks, CEO of Eli Lilly and Mr. Darren Carroll, Senior Vice President of Corporate Business Development, said in an interview at the JP Morgan Healthcare Conference in San Francisco, California, that the recent US tax reform The program gives the company more flexibility in the allocation of funds. Additional funding will make the company more competitive in purchasing assets and provide some room for manoeuvre in infrastructure construction and growth. In an interview, Mr. Ricks said: "What is the next investment, where is the next factory built, what to study in the next lab, we can not worry about the tax rate when making decisions about these issues, but only care Where are our talents and markets, and let go." On the acquisition side, the company plans to expand its immunology and oncology. Mr. Carroll pointed out that as far as oncology is concerned, "we will choose the most appropriate one with a one-size-fits-all attitude."

Currently, there is an abemaciclib in the Lilly's oncology pipeline that is undergoing FDA review. Abemaciclib is a cyclin-dependent kinase inhibitor that targets CDK4 and CDK6 for the treatment of metastatic breast cancer. In October last year, the FDA granted abemaciclib a priority review. Now, abemaciclib is also undergoing clinical phase 3 trials as breast cancer adjuvant therapy and lung cancer therapy, as well as clinical phase 2 trials in pancreatic cancer and non-small cell lung cancer.
Oncology drugs in clinical phase 3 trials include ramucirumab (LY3009806), an anti-angiogenic monoclonal antibody that is currently being tested and evaluated in patients with bladder cancer, gastric cancer, non-small cell lung cancer, and liver cancer. In addition, there are PI3 Kinase/mTOR dual inhibitor LY3023414 in clinical phase 2 trial, and Chk1 inhibitor prexasertib (LY2606368) in clinical phase 2. Lilly also has a combination of PD-L1 antibody drug and ritual drugs, which is still in clinical phase 1, and TGF-ß Receptor I kinase inhibitors.

â–² Lilly is a new oncology research drug located in Phase 3 clinical phase (Source: Eli Lilly Official Website)
In immunology, Lilly has a drug Taltz / ixekizumab (LY2439821) for neutralizing soluble interleukin-17A (IL-17). Taltz received FDA approval for psoriasis in 2016, a chronic immune disease that affects the skin. At present, Eli Lilly is evaluating the efficacy of this drug for the treatment of axial spondyloarthritis in clinical phase 3 trials. In addition, Lilly also has baricitinib for the treatment of arthritis, which is awaiting FDA approval.
Another area of ​​interest to Lilly is pain management and related fields. Galcanezumab (LY2951742), an administrative review stage, is a monoclonal antibody to CGRP that binds to and inhibits calcitonin gene-related peptide (CGRP) activity. Prevention of chronic and sporadic migraine in adult patients. Currently, the drug is also evaluated for the effectiveness of a cluster headache in a clinical phase 3 trial. Another Lilly-treated migraine drug is clinically stage 3 lasmiditan (LY573144), an oral 5-HT1F receptor agonist for acute treatment of migraine. The company also has clinical phase 3, tanezumab, a humanized monoclonal antibody that inhibits nerve growth factor, and is currently working with Pfizer to study a variety of pain indications, including osteoarthritis pain. The effects of cancer-induced pain, long-term back pain, and the like. Tanezumab was awarded the fast track qualification by the US FDA last year.
An important part of Lilly's R&D pipeline is diabetes and high blood pressure drugs. At present, Lilly has a nasal glucagon in clinical phase 3 trials for the treatment of severe hypoglycemia in diabetic patients. Also in clinical phase 3 is the ultra-fast insulin LY900014, which is used to treat patients with type 1 and type 2 diabetes. It contains a new formulation to accelerate the absorption of insulin.
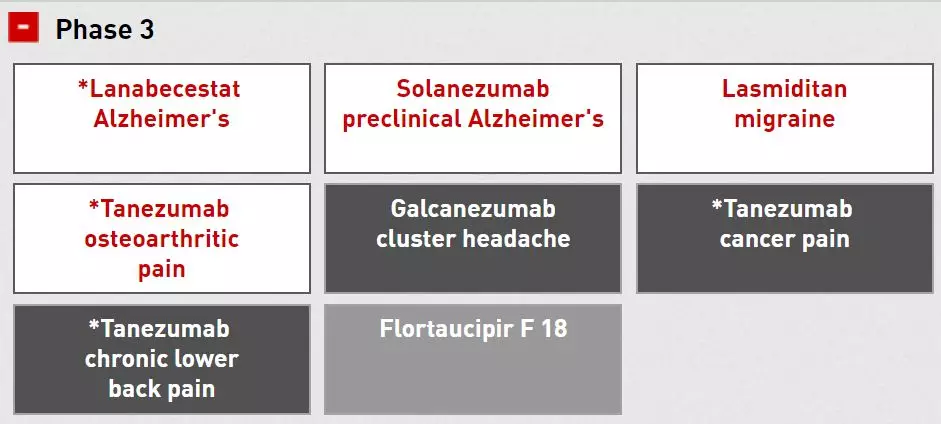
â–²Lilly is a new drug in the field of neurology in the clinical phase of Phase 3 (Source: Eli Lilly Official Website)
Alzheimer's Disease (AD) is also part of the Lilly pipeline. Lilly has a clinical stage 3 lanabecestat (LY3314814, formerly known as BACE-AZD3293), which is an oral beta secretase cleaving enzyme (BACE) inhibitor for AD treatment. Solanezumab (LY2062430), also in clinical stage 3, is used to delay progression of AD, which binds to β-amyloid (Aβ), a soluble monomeric form, and Aβ deposition in the brain is typical of AD patients. The cause of targeting Aβ by many therapies. In addition, Lilly also has Flortaucipir F-18, which is a reagent for the pathology diagnosis of Tau in PET imaging. Tau protein entanglement is also an important feature of AD patients.
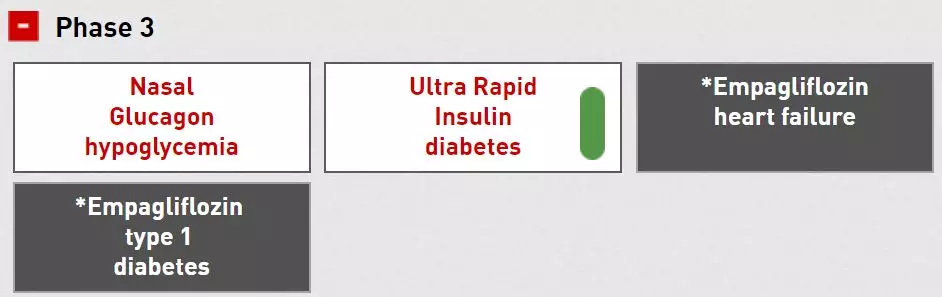
â–²Lilly is a new drug for diabetes research in Phase 3 clinical phase (Source: Eli Lilly Official Website)
The drug in the Phase III trial phase of the Lilly line is also empagliflozin. Empagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor developed by Lilly and Boehringer Ingelheim. SGLT2 is the major transporter that reabsorbs glucose back into the bloodstream in the glomerulus. By inhibiting the action of SGLT2, empagliflozin reduces the reabsorption of glucose in the kidneys, allowing more glucose to escape through the urine, thereby lowering the level of glucose in the blood. Empagliflozin has received FDA approval at the end of 2016 to reduce the risk of death from cardiovascular disease in patients with type 2 diabetes who have cardiovascular disease.
In addition to the above, Lilly's research and development pipeline also has a number of drugs still in clinical phase 1 and phase 2, covering immunology, cancer, nervous system diseases, pain management, diabetes and other fields.
Reference materials:
[1] Eli Lilly on the Hunt for Cancer Deals to Beef Up Pipeline
[2] Lilly official website
The Anti Rabies Vaccine For Human vaccine can prevent rabies. Rabies is a serious illness caused by a virus. The rabies virus is spread to humans through the bite or scratch of an infected animal. Dogs, bats, skunks, coyotes, raccoons, and foxes are examples of animals that can carry rabies. The rabies vaccine can protect you from infection if you are at high risk of exposure. The vaccine can also prevent infection after you are bitten by an infected animal.
Anti Rabies Vaccine For Human,Rabies Vaccine Dose,Human Freeze Anti Rabies Vaccine,Rabies Vaccine Vero Cell For Human
Changchun Zhuoyi Biological Co., Ltd , https://www.zhuoyi-bio.com