(1) Application range
The GyrolabxPlore fully automated upgrade immunoassay workstation from Gyros AB of Sweden is a new product developed by the high-throughput GyrolabxP workstation for medium and low-throughput laboratories. GyrolabxPlore uses a special microfluidic CD as a consumable for immune response, which automatically completes 112 samples of immune response in one hour and obtains high quality data. The product has been awarded the "2015 Frost & Sullivan's North American Immunoassay Market New Product Innovation Award".
It is suitable for pharmacokinetics (PK: Pharmacokinetics), pharmacodynamics (PD: Pharmacodynamics), kinetics (TK: Toxicokinetics), immunization of macromolecules for early development/preclinical/clinical/post-market surveillance. Immunogenicity, Biomarker Monitoring, ADA: Anti-drug Antibody, IgG titer, Protein A assay, Host Cell Protein (HCP) . It can replace the traditional ELISA reaction and has many advantages over ELISA, such as less sample loading than ELISA (nano upgrade), wider detection range, higher sensitivity and better precision. The internal structure of GyrolabxPlore is shown in Figure 1.

Figure 1. Internal structure of GyrolabxPlore
Figure 1 identifies the description:
1: a robot arm for positioning the sampling needle and the detector;
2: Wash the needle groove to ensure no cross contamination;
3: Rotating disc, can put 1 CD, can accurately control the liquid flow in the CD and perform high-sensitivity detection;
4: High sensitivity laser induced fluorescence detector mounted on the robot arm;
5: 10 sampling needles, which can accurately transfer samples and reagents from the microplate to the CD;
6: Microplate slot for 3 microplates for loading samples, reagents and standards.
(2) Working principle
GyrolabxPlore uses special microfluidic CD consumables, and all reagents and samples are loaded, adsorbed, eluted and tested on the CD. General PK, PD reactions were performed on a Gyrolab Bioaffy CD. Taking the Bioaffy 200 CD as an example, the GyrolabxPlore is equipped with a robotic arm that uses a pipetting needle to transfer samples and reagents from the microplate to each microstructure in the CD. The CD microstructure contains 15nl of streptavidin. With the microbead affinity column coated with the prime, the CD is precisely rotated at the appropriate speed and time to ensure that the sample and reagent flow in parallel through the affinity column for reaction. The microbeads are first combined with the biotin capture reagent, and the capture reagent is specifically bound to the sample to be tested, and finally the secondary antibody with the fluorophore is attached to form a "streptavidin microbead-biotin capture reagent-sample. - a complex of a secondary anti-fluorescence detection reagent". The fluorescence reading is excited by a laser. The entire process is fully automated by Gyrolab xPlore, and it takes only one hour to fully automate the detection of one CD 112 data points.

Figure 2. Left: Microstructure of the Gyrolab Bioaffy 200 CD; Right: Microstructure of the Gyrolab Mixing CD
Figure 2 identifies the description:
1: general reagent pipeline;
2: Hydrophobic barrier, which can block the flow and ensure the consistency of liquid quantification;
3: affinity column (15nL streptavidin coated beads);
4: reagent volume quantitative area (200 nL);
5: The sample is added to the mouth;
6: Volumetric chamber for accurate quantification of sample volume (20,200 or 1000nL for Bioaffy CD, 200nL for Bioaffy 200, 200nL for Mixing CD);
7: The overflow line of excess liquid ensures that the liquid fills the dosing chamber.
8: mixing the reaction chamber;

Figure 3. Complex of "streptavidin microbead-biotin capture reagent-sample-secondary antibody fluorescence detection reagent"
Gyrolab Bioaffy CD model for routine experimentation:
(1) Bioaffy 20HC: loading volume: 20nL, data points: 112;
(2) Bioaffy 200: loading volume: 200 nL, data points: 112;
(3) Bioaffy 1000: loading volume: 1000 nL, data points: 96.
The CD for anti-drug antibody (ADA) detection and Protein A detection is Gyrolab Mixing CD. Taking ADA as an example, Gyrolab's ADA treatments fall into two categories: long-term equilibration without acid dissociation by Bioaffy 200 CD, and acidification neutralization using acid dissociation by Gyrolab Mixing CD. In the acidification neutralization method, the complex of the drug and ADA reacts with the acidic buffer to cause dissociation, and the ADA is released. Neutralization with a neutralizing buffer containing a biotin-labeled drug and a fluorescein-labeled drug. The formed "biotin drug-ADA-fluorescent labeling drug" complex is bound to the affinity column by "streptavidin-biotin" action, and the fluorescence is excited by a laser to complete the detection of the fluorescence value of the sample. 48 samples can be tested in one hour.
Taking the Protein A assay as an example, a biotin-containing capture reagent is first combined with an affinity column. The complex of IgG and Protein A reacts with the acidic buffer, dissociation occurs, and Protein A is released and bound to the capture reagent. Finally, a secondary antibody fluorescent labeling detection reagent is added to form a complex of "streptavidin microbead-biotin capture reagent-Protein A-secondary fluorescent detection reagent". The fluorescence is excited by a laser to complete the detection of the fluorescence value of the sample. 48 samples can be tested in 80 minutes.
The workstation uses Gyrolab Evaluator software to evaluate the results or export the results to other software such as the LIMS system. The software complies with 21 CFR part 11 of the US Federal Regulations and provides extensive verification support.
(3) Comparison and advantages with traditional ELISA
Traditional ELISA has many deficiencies, such as large sample size, long reaction time, narrow dynamic range, poor reproducibility, inability to automate, and labor. GyrolabxPlore can do ELISA work, but it has many advantages over ELISA, such as: less sample loading than ELISA (nano upgrade), wider detection range, higher sensitivity and better precision. The product advantages are as follows:
(1) Save time: get results in one hour, develop new experiments in a few days instead of weeks;
(2) Saving sample size: the sample loading of nano-upgrading (minimum sample loading 20nl), reducing the consumption of experimental animals, can achieve continuous sampling of one mouse, and obtain a set of macromolecular drug metabolism data;
(3) Saving labor: fully automatic operation, can automatically complete the loading, adsorption, elution, detection and data analysis of 112/96 samples;
(4) Wide dynamic range: Minimize dilution and repeatability, capture reaction system through 15nl affinity column sample, effectively improve data dynamic range and reduce matrix effect.
(5) Flexibility: Using the same CD consumables and similar software as the high-throughput GyrolabxP workstation, the experimental method is easy to transfer between different instruments and locations, and can guarantee the high quality of the data.
A comparison of the Gyrolab xPlore workstation with the traditional ELISA method is shown in Table 1:
Table 1. Comparison of Gyrolab xPlore workstations with traditional ELISA methods: data from Pfizer
parameter | Gyrolab xPlore | ELISA | Gyros improvements |
Microplate liquid volume | 8μL | 100μL | 10 times smaller volume |
Experiment run time (including coating time) | <1 hour | 10 hours | 10 times shorter running time |
Dynamic Range | 10.5-6400ng/ml | 88-666ng/ml | Dynamic range is expanded by 8 times |
Minimum dilution factor MRD | 1:2 | 1:20 | MRD is reduced by 10 times |
Experimental development time | 3 days | 1 week | Development speed increased by 2.3 times |
Can it be automated? | can | no | Built-in automation equipment |
Whether it is affected by desktop experiments | no | Yes | Not affected by desktop experiments |
(4) Specific application examples
(1) Pharmacokinetic analysis
Drug metabolism and kinetic studies of candidate biopharmaceuticals (DMPK) are a challenge for microvolume bioanalysis. GyrolabxPlore runs an upgraded immune response that produces a complete PK curve from a mouse, as shown in Figure 4. Gyros technology is based on the capture of samples with an affinity column, which minimizes matrix interference and significantly reduces the minimum dilution factor MRD. See Figure 5, which shows that the standard curve of undiluted serum and 1:4 diluted serum almost coincides. No need to dilute the sample.
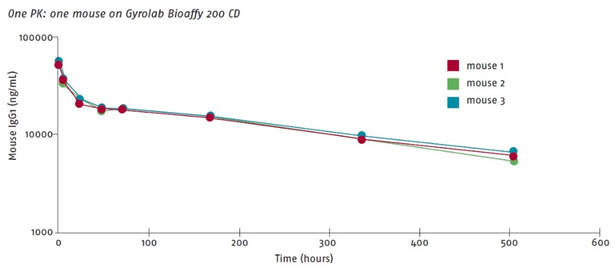
Figure 4. Three complete PK curves from three mice (intravenous therapeutic antibody): data from AstraZeneca

Figure 5. Standard curve of drug molecules derived from undiluted serum (green) and 1:4 diluted serum (blue)
(2) ADA detection
Immunogenicity testing is one of the necessary routine steps for biopharmaceutical safety testing. The ADA (Anti-drug Antibody) immunization experiment is very complicated, subject to drug tolerance and sensitivity, and requires a lot of manpower, and a long experimental period to take the drug. Maximizing tolerance, minimizing drug interference, requires overnight incubation and acid sample pretreatment. Gyrolab's ADA treatments fall into two categories: long-term equilibration without acid dissociation via Bioaffy 200 CD, and acidification neutralization with acid dissociation via Gyrolab Mixing CD (see (2) for details) ). The drug tolerance of the two methods is shown in Figure 6. The left image shows the ADA test with the Bioaffy 200 CD, and the right image shows the ADA test with the Gyrolab Mixing CD. The method on the left does not require acidic dissociation and has a low tolerance to free drug concentrations, suggesting that an acidic dissociation step may be required. When 10 μg/ml of unlabeled drug is present, 250 ng/ml of ADA can be detected. The method on the right includes acidic dissociation, which increases tolerance to free drug and can detect 250 ng/ml of ADA in the presence of up to 100 μg/ml unlabeled drug. The advantages of the Gyrolab method are: automatic sample pretreatment, reducing manual operation time; greatly shortening the experiment time and completing it within one hour; saving precious reagents and samples, only 5 μL of sample can be double-repetitive; there is a special ADA software and ADA buffer is available.
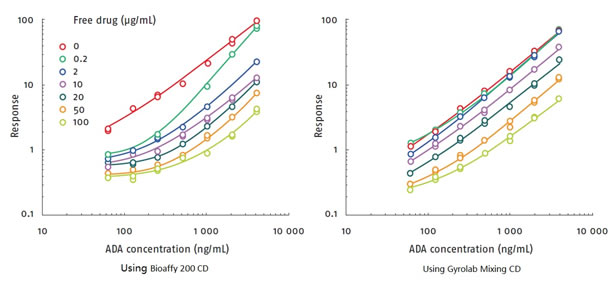
Figure 6. The left image shows the ADA assay with the Bioaffy 200 CD, and the right panel shows the ADA assay with the Gyrolab Mixing CD.
(3) HCP impurity detection
Host cell protein (HCP) impurities are immunogenic, so they are mandatory items in the development and production of biopharmaceuticals. Rapid and accurate quantification of drugs and HCP impurities is key to the effective development and optimization of bioprocesses. Current techniques, such as ELISA and HPLC, yield better quality results but have deficiencies in flux and dynamic range. Gyrolab offers significant advantages over ELISA, such as: a wider dynamic range; faster experimentation; better precision and repeatability and automation.
(a) Dynamic range: Figure 7 compares the results of detection of HCP impurities by Gyrolab and ELISA. Compared to the ELISA method, Gyrolab has a dynamic range of 100 times larger.

Figure 7. Comparison of dynamic range between ELISA and Gyrolab: data from Merck
(b) Accuracy: Figure 8 shows that both methods show the same experimental results in various purification steps.

Figure 8. Comparison of HCP concentrations detected by ELISA and Gyrolab in various purification steps
(c) Precision: Figure 9 shows a comparison of the two methods for analysis of samples from various purification steps. The error bar shows that the data obtained from Gyrolab has less variation and higher precision.
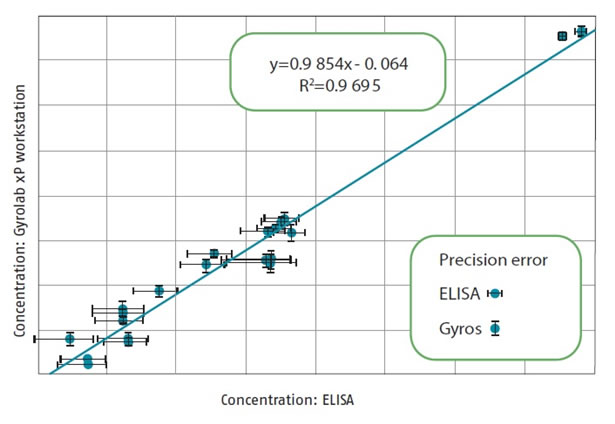
Figure 9. Analysis of a sample of the purification process of a therapeutic protein: data from MedImmune
(d) Selectivity: Table 2 shows that the two samples were processed at different dilutions (HCP is expected to be approximately 10,000 ng/ml), and the detected HCP concentrations have CV values ​​less than 10% (double replicates), showing polar Good dilution linearity with a minimum dilution factor of 2 times.
Table 2. CV values ​​of HCP concentrations for samples at different dilution factors
(e) Comparison and advantages with ELISA methods: see Table 3, data from MedImmune and Merck. 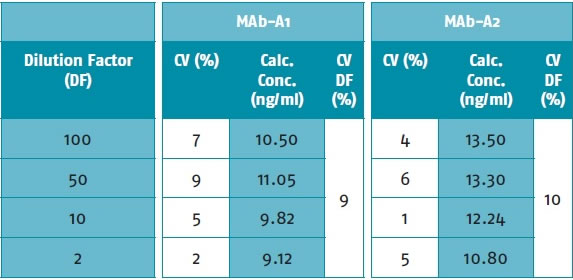
Table 3. Comparison of GCP detection by Gyrolab and ELISA

(4) Protein A impurity detection
Protein A binds to human IgG and is the key to affinity purification of biotherapeutic products. However, Protein A will detach from the chromatography resin and be eluted with the therapeutic antibody product, becoming an impurity in the bioprocess. Therefore, it is very important to accurately and quantitatively detect Protein A during the purification process. Performing a Protein A test on an untreated sample underestimates the degree of contamination because IgG will occupy the epitope of Protein A, so the sample must be dissociated by Protein A and IgG. In an automated method of quantifying residual Protein A ligands with Gyrolab, the sample and acidic buffer were reacted in a Gyrolab Mixing CD to dissociate the complex of Protein A and IgG. Protein A was then tested by sandwich immunoassay. See (2) Working principle for details. Detection of Protein A impurities requires the use of Gyros' Protein A kit.
(a) Specificity: Three Protein A derived from USP were accurately quantified using the Gyrolab Protein A kit. The three are: native Protein A; recombinant Protein A (E. coli) and recombinant Protein A, C-Cys (MabSelectSuRe), see Figure 10.
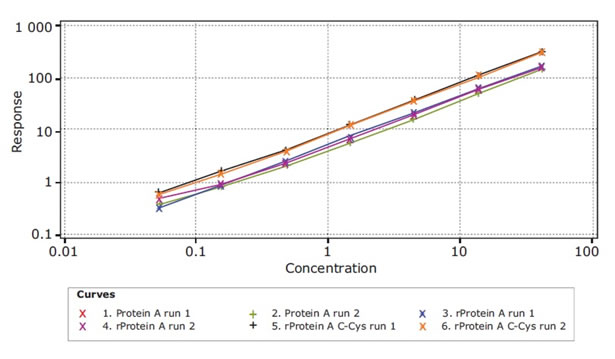
Figure 10. Specificity of a series of Protein A molecules
(b) Accuracy: The internal standards of Protein A and MabSelect SuReligand were performed on four commercial antibodies, and the recovery of internal standard was within an acceptable range (±20%, n=2), see Table 4.
Table 4. Recovery of Protein A internal standard

(c) Precision and repeatability: The data is a natural Protein A standard curve showing a small CV. The standard curve range is: 0.055-40 ng/ml. See Table 5. Figure 11 shows three consecutive standard curves showing excellent linearity and repeatability.
Table 5. Natural Protein A Standard Curve
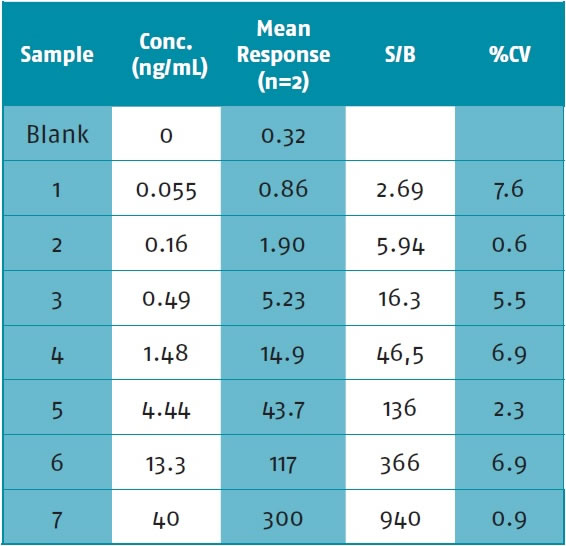

Figure 11. Three consecutive standard curves
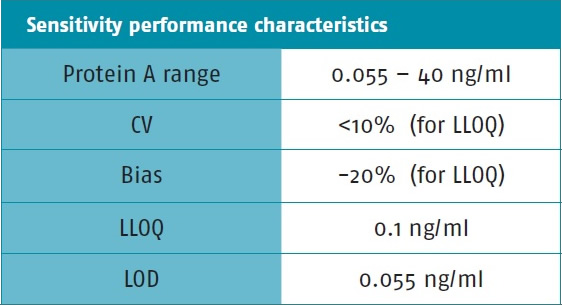
(d) Sensitivity: Sensitivity assay A commercial therapeutic monoclonal antibody diluted to 0.5 mg/ml was used and the internal standard Protein A was added. See Table 6.
Table 6. Sensitivity testing
(e) Comparison and advantages with ELISA methods: see Table 7, data from Cobra Biologics, Sweden.
Table 7. Comparison of Gyrolab and ELISA for Protein A detection
Gyrolab Workstation and Gyrolab Protein A Kit | Commercial ELISA Kit | |
Sample preparation | 30 min | 45min |
Sample processing - acidification | Automatically | Manually |
Sample consumption | 10μL | 500μL |
IgG tolerance | 0.5 mg/ml | 0.125 mg/ml |
Dynamic Range | 0.055-40 ng/ml | 0.05-1.6 ng/ml |
Experimental run time | 80 min/CD | 90 min / board |
The amount of data that runs once | 48 /CD | 96-well plates |
Flux | 10 CD/day (automatic) | 5 boards / day (manual) |
Sensitivity (LLOQ) | Ng/ml ppm | Ng/ml 0.8 ppm |
Whether it is interfered by other experiments | no | Yes |
(f) Summary: Gyrolab can quantify residual natural Protein A, recombinant Protein A variant and MabSelectSuRe. High quality data in 80min, with high sensitivity, accuracy, accuracy and repeatability. Gyrolab's solution is overall superior to ELISA. The Protein A kit is easy to use, and the automated sample acidification process reduces manual steps and time, reduces the risk of errors, and produces consistent data results.
(5) Summary
GyrolabxPlore automates the immune response in a nanoscale microfluidic structure in CD format. Each microstructure on the CD contains a 15nl affinity column preloaded with streptavidin coated beads, supporting a variety of immunoassays, including antibody sandwich methods and indirect antibody methods. Compared to the ELISA method using a microplate, it significantly reduces the consumption of samples and reagents. Microfluidic technology ensures that all samples on the CD can be processed in parallel, producing consistent results. Each microstructure is equivalent to one data point, eliminating cross-contamination. The product's control and analysis software complies with 21 CFR part 11 regulations, ensuring that experiments can be developed and transferred in both GMP and GLP environments. For bioprocesses and production processes, miniaturized reaction times, faster reaction times, broader dynamic range and excellent accuracy, precision and repeatability make Gyrolab products the ideal immune response tool to support biotechnology And the various processes of production.
The Gyrolab series has entered many biopharmaceutical companies, CROs, CMOs and R&D institutions, and some existing customers are shown in Figure 12.

Figure 12. Existing customers of Gyrolab products
Distributor of Greater China: Shanghai Dianao Biotechnology Co., Ltd. For more information, please refer to the Gyros website: and Shanghai Dian Biotech Co., Ltd. website: , or follow the WeChat public account "Diao Biotech": tekontech.
Product introduction link:
â— medium and low throughput GyrolabxPlore (1 CD) ;
â— High-throughput GyrolabxP workstation (5 CDs) .
For product inquiries, please contact Manager Liu of the Marketing Department, Tel: (+86) 21-58605185, Fax: (+86) 21-51973282, E-mail:
12V Fascial massage gun supply about 8mm deep muscle tissue relaxation, it is professional massage gun. 12v fascia massage gun have 6pcs massage head ( Fork, Ball, Flat,Bullet,finger, y-shape). We are a China professional manufacturer. We can do 7.4V,12V,16.8V,24V and Mini. Massage Gun. Our products quality is great. If you are interested in our products, welcome to contact us.
wellness massage gun, vibration massage gun,cheap massage gun, sport massage gun
Ningbo Prestigeplus Commodity Co.,Ltd , https://www.prestigemedics.com